Last Updated: February 13, 2026
Table of Contents
- Introduction
- Understanding Tablet Sticking: Definitions and Impact
- Quick Diagnostic Framework
- Formulation-Related Causes and Solutions
- Tooling-Related Solutions
- Process Parameter Optimization
- Environmental Control Strategies
- Advanced Solutions and Technologies
- Solution Selection Framework
- Case Studies: Real-World Problem Solving
- Prevention Strategies During Development
- Monitoring and Continuous Improvement
- Common Questions About Tablet Sticking
- What is the most common cause of tablet sticking?
- Can I fix sticking without reformulating?
- How do I know if I need to recoat my tooling?
- What’s the difference between chromium nitride and titanium nitride coatings?
- How much magnesium stearate is too much?
- Should I increase or decrease compression force when experiencing sticking?
- How does humidity affect tablet sticking?
- Can tablet design contribute to sticking?
- When should I use a coating on my punches?
- How long do anti-stick coatings last?
- What are the signs that granules are too moist?
- Can environmental temperature cause sticking?
- Key Takeaways and Action Steps
Introduction
Tablet sticking remains one of the most persistent and costly problems in pharmaceutical manufacturing. When granulated powder adheres to punch faces during compression, the consequences cascade rapidly: production halts for emergency cleaning, batch yields plummet, quality defects multiply, and manufacturing timelines slip. For pharmaceutical manufacturers, formulators, and production managers, the pressure to identify root causes and implement effective solutions is immediate and intense.
The challenge with tablet sticking lies in its multifactorial nature. A sticking problem might originate from formulation composition, tooling surface characteristics, compression parameters, environmental conditions, or—most frustratingly—a combination of these factors. Trial-and-error troubleshooting wastes valuable production time and resources, while incorrect diagnosis can actually worsen the problem.
This comprehensive guide provides what pharmaceutical professionals need most: a systematic, diagnostic-first approach to identifying and resolving tablet sticking problems. Whether you’re facing a production crisis requiring immediate intervention or developing preventive strategies for new formulations, you’ll find structured frameworks, specific technical parameters, and evidence-based solutions throughout this resource.
Unlike generic troubleshooting advice, this guide integrates formulation science, tooling technology, and process optimization into cohesive decision-making frameworks. You’ll learn how to rapidly diagnose whether your sticking problem requires formulation adjustment, tooling modification, process optimization, or environmental control—and exactly how to implement each solution category effectively.
Understanding Tablet Sticking: Definitions and Impact
What is Tablet Sticking?
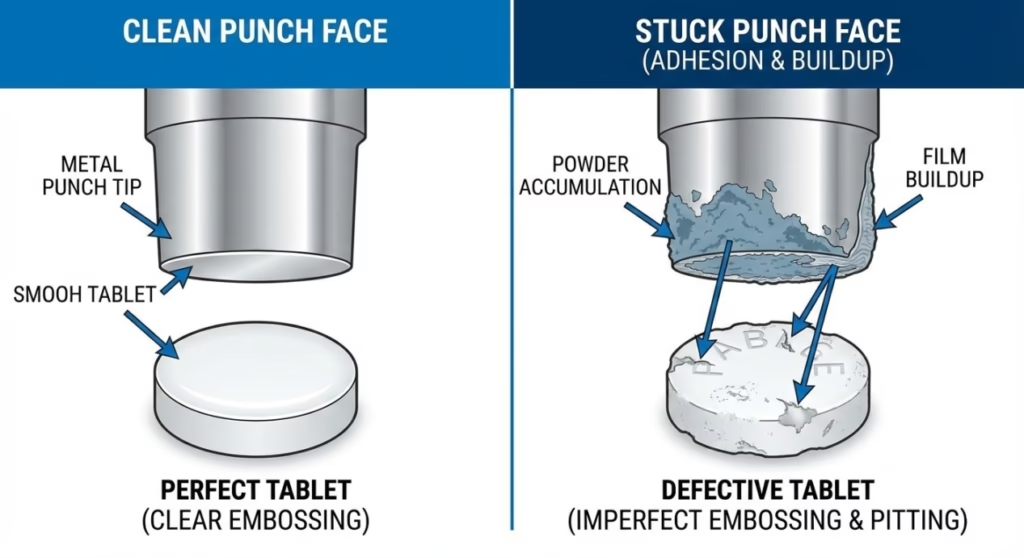
Tablet sticking, also called filming, occurs when tablet material adheres to the punch face during compression rather than releasing cleanly during ejection. This adhesion manifests as powder buildup on punch tip surfaces, resulting in tablets with surface defects, incomplete embossing, or visible material removal.
The sticking mechanism involves adhesive forces between the formulation and tooling surface that exceed the cohesive forces holding the tablet together. When a tablet is ejected from the die, these stronger adhesive forces cause partial tablet material to remain on the punch face rather than staying with the intact tablet. With each subsequent compression cycle, additional material accumulates on the punch, creating increasingly severe defects until production must stop for punch cleaning.
Visual identification of sticking is straightforward: inspect punch faces after compression cycles for powder accumulation, film formation, or granule buildup. Early-stage sticking appears as light powder dusting on punch tips, while advanced sticking shows thick material layers that clearly transfer to tablet surfaces.
Sticking vs. Picking: Critical Differences
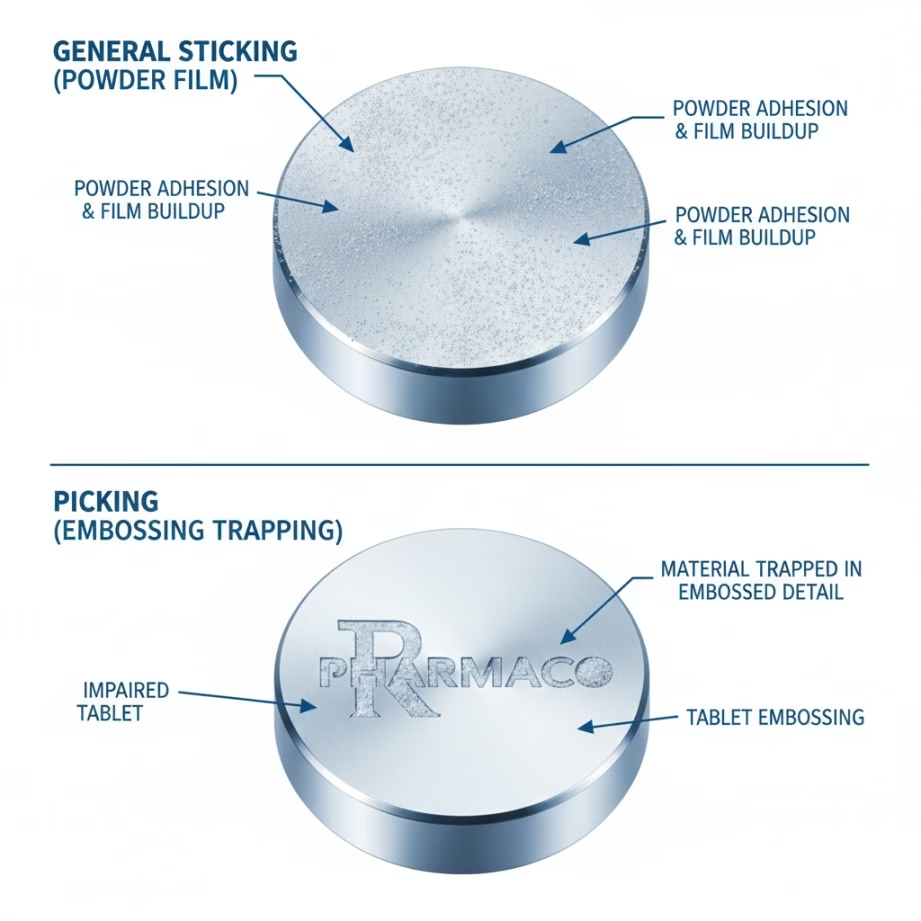
While often discussed together, sticking and picking represent distinct phenomena requiring different solutions.
Picking specifically refers to material adhering to embossed areas of punch faces—letters, logos, numbers, or other engraved features. During compression, powder fills these recessed areas and adheres rather than ejecting with the tablet. The result is “picked out” sections on the tablet face where embossed features should appear, and corresponding powder buildup in punch engravings.
Sticking occurs across the broader punch face surface, not limited to embossed features. Material adheres to the general cup area, edges, or entire punch tip.
This distinction matters because solutions differ:
- Picking often requires tablet design modifications (font selection, engraving depth, pre-picking techniques) or specialized coatings
- General sticking typically responds to formulation optimization, lubrication adjustment, or overall surface treatments
Many formulations exhibit both phenomena simultaneously, requiring integrated solutions addressing both mechanisms.
The Real Cost of Tablet Sticking
The economic impact of tablet sticking extends far beyond obvious production downtime:
Direct Production Losses:
- Emergency production stops for punch cleaning (15-45 minutes per incident)
- Reduced compression speeds to mitigate sticking
- Batch rejection when defect rates exceed specifications
- Operator time diverted to frequent tooling inspection and maintenance
Quality and Compliance Impact:
- Tablet appearance defects affecting patient acceptance
- Incomplete embossing creating potential product identification issues
- Increased variability in tablet weight and hardness
- Documentation burden for deviations and investigations
Equipment and Tooling Wear:
- Accelerated punch face degradation from aggressive cleaning
- Increased tooling replacement frequency
- Potential damage to tablet press surfaces from excessive force during sticking
- Higher maintenance costs for compression equipment
Development and Scale-Up Delays:
- Extended formulation optimization timelines
- Unexpected problems during technology transfer
- Additional stability studies if reformulation required
- Delayed market entry for new products
Understanding these cascading costs underscores why rapid, accurate diagnosis and effective solution implementation represent critical capabilities for pharmaceutical manufacturers.
Quick Diagnostic Framework
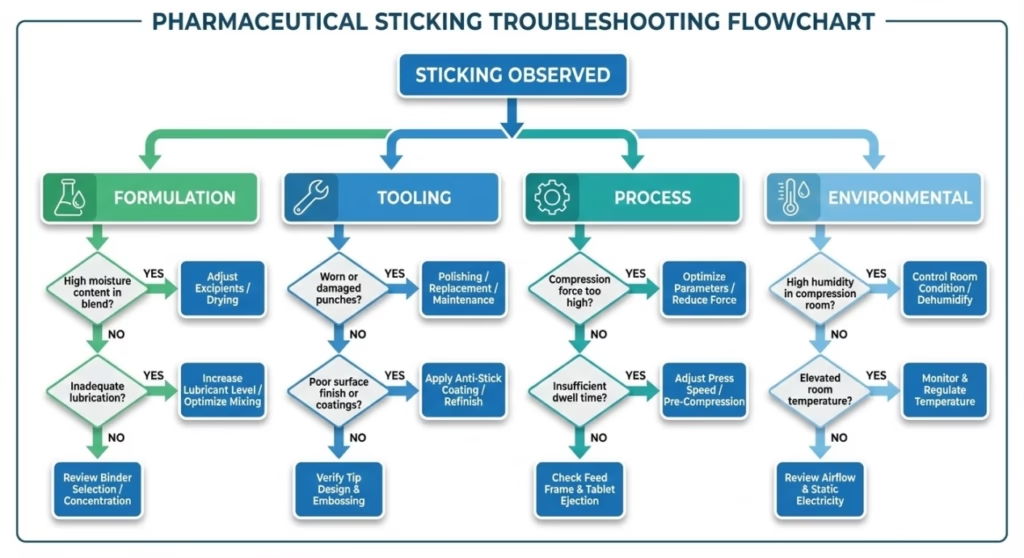
Effective troubleshooting begins with accurate diagnosis. Rather than implementing random solutions, use this systematic framework to identify the root cause category before selecting interventions.
Symptom-to-Cause Mapping Table
[Visual Table: Observable Symptoms → Likely Root Causes]
| Observable Symptom | Primary Suspect | Secondary Considerations |
|---|---|---|
| Powder accumulation on entire punch face | Insufficient lubrication | Excess moisture, compression speed too fast |
| Material buildup in embossed areas only | Picking (design issue) | Font complexity, insufficient lubricant distribution |
| Sticking worse in humid conditions | Hygroscopic formulation/moisture | Environmental control inadequate |
| Sticking increases throughout batch | Granule moisture too high | Inadequate drying, hygroscopic API |
| Inconsistent sticking across turret | Lubricant mixing time insufficient | Uneven lubricant distribution, tooling wear variation |
| Sudden onset after tooling change | Punch surface finish/coating | Tooling specification mismatch |
| Sticking worse with compression speed increases | Dwell time sensitivity | Formulation plastic/elastic properties |
| Tablets soft with excessive sticking | Insufficient compression force | Weak granules, inadequate binder |
| Sticking accompanies tablet capping | Excessive compression force | Air entrapment, granule too hard |
| Film buildup with oily appearance | Oily/waxy excipients | Low melting point materials, temperature too high |
The 5-Minute Initial Assessment
When sticking occurs during production, gather these critical data points immediately before stopping the press:
Visual Inspection Checklist:
- [ ] Photograph punch faces showing sticking pattern
- [ ] Note whether sticking is uniform across all stations or isolated
- [ ] Observe whether upper punches, lower punches, or both are affected
- [ ] Check if sticking occurs on cup faces, edges, or embossed areas specifically
- [ ] Inspect tablets for corresponding defects (material removal, incomplete embossing)
Process Parameter Documentation:
- [ ] Record current compression speed (tablets per minute)
- [ ] Note compression force settings and actual force readings
- [ ] Document any recent changes to process parameters
- [ ] Record environmental conditions (temperature, relative humidity)
- [ ] Note position in batch (beginning, middle, end)
Material Observation:
- [ ] Collect granulation sample for moisture testing
- [ ] Assess granule flow characteristics visually
- [ ] Check for any formulation changes or new material lots
- [ ] Verify lubricant addition and mixing time records
- [ ] Note granule appearance (dry, moist, oily)
Quick Tests (Without Stopping Production):
- Reduce compression speed by 20-30% and observe if sticking improves
- Verify environmental control systems are functioning
- Check tooling maintenance logs for cleaning/replacement history
- Review formulation records for recent changes
This rapid assessment provides the foundation for accurate diagnosis without extensive downtime.
Determining Root Cause Category
Use this decision framework to categorize your sticking problem:
Is it Formulation-Related?
Indicators pointing to formulation as primary cause:
- Sticking occurs with new or modified formulations
- Problem appeared after raw material supplier change
- Consistent sticking across all tooling sets
- Moisture testing reveals out-of-specification values
- Granule properties visibly different from normal
- Historical data shows this formulation prone to sticking
- Environmental conditions within normal range
Is it Tooling-Related?
Indicators pointing to tooling as primary cause:
- Sticking began after tooling change or maintenance
- Problem isolated to specific punch stations
- Microscopy reveals surface damage or coating wear
- New tooling design with complex embossing
- Historical success with same formulation using different tooling
- Punch face appearance shows wear patterns
- Formulation and process parameters unchanged
Is it Process-Related?
Indicators pointing to process parameters as primary cause:
- Recent changes to compression speed or force
- Sticking correlates with production rate increases
- Problem varies by time of day or shift
- Inconsistent sticking throughout batch
- Equipment recently serviced or adjusted
- Operator technique variations noted
- Formulation and tooling historically successful
Is it Environmental?
Indicators pointing to environmental factors as primary cause:
- Sticking varies with weather or seasons
- Problem worse during high humidity periods
- Temperature fluctuations in compression area
- HVAC system changes or malfunctions
- Worse in certain manufacturing locations
- Correlates with time of year
- Same formulation/tooling successful in different environment
Multiple Factor Situations:
Most persistent sticking problems involve multiple contributing factors. If indicators point to more than one category, prioritize solutions as follows:
- First: Address process and environmental factors (fastest, lowest cost)
- Second: Optimize formulation within specification limits (lubrication, moisture)
- Third: Consider tooling modifications (coating, surface treatment)
- Fourth: Reformulation or major design changes (most expensive, slowest)
Formulation-Related Causes and Solutions
Formulation composition represents the most common root cause of tablet sticking and often provides the most sustainable long-term solutions.
Binder Issues
Binders provide cohesiveness to powders during compression, but incorrect binder selection or concentration directly impacts sticking propensity.
Too Much Binder:
Excess binder creates overly cohesive, sticky granules that readily adhere to punch surfaces. Signs include:
- Granules appear dense and hard
- Tablets have excessive hardness
- Sticky residue visible on punch faces
- Difficulty achieving target dissolution rates
Solutions:
- Reduce binder concentration incrementally (typically 0.5-1.0% reductions)
- Test each adjustment to verify maintained tablet integrity
- Consider switching to less adhesive binder alternatives
- Optimize granulation liquid addition rate
Common binder concentrations by type:
- Povidone (PVP): 1-5% of formulation weight
- Hydroxypropyl cellulose (HPC): 2-5%
- Hydroxypropyl methylcellulose (HPMC): 2-5%
- Microcrystalline cellulose (MCC): 10-30% (also functions as filler)
- Starch paste: 5-10%
Insufficient Binder:
While less common as a sticking cause, inadequate binder can create weak granules that fracture during compression, leaving particles adhering to punch surfaces rather than cohering into intact tablets.
Solutions:
- Increase binder concentration incrementally
- Improve granulation technique to maximize binder efficiency
- Ensure adequate wetting during granulation process
- Verify binder dissolution in granulating fluid
Binder Selection Criteria:
Different binders exhibit varying adhesive characteristics:
- Low sticking propensity: Microcrystalline cellulose, pregelatinized starch
- Moderate sticking propensity: Hydroxypropyl cellulose, HPMC
- Higher sticking propensity: Povidone (especially at high concentrations), gelatin
When reformulation is possible, selecting binders with lower inherent tackiness reduces sticking risk while maintaining required binding function.
Lubrication Problems

Proper lubrication is critical for preventing sticking. Lubricants reduce friction between tablet material and punch/die surfaces, facilitating clean ejection.
Insufficient Lubrication:
Inadequate lubricant quantity represents one of the most frequent causes of tablet sticking. Symptoms include:
- Progressive sticking worsening throughout batch
- High ejection forces
- Tablet edges showing abrasion
- Powder film accumulating on punch faces
Optimal Lubricant Concentrations:
| Lubricant Type | Typical Range | Common Optimal | Notes |
|---|---|---|---|
| Magnesium stearate | 0.25-1.0% | 0.5-0.75% | Most widely used; higher concentrations may reduce dissolution |
| Stearic acid | 1.0-2.5% | 1.5-2.0% | Effective but higher concentration needed |
| Sodium stearyl fumarate | 0.5-2.0% | 1.0% | Moisture-sensitive formulations; less impact on dissolution |
| Calcium stearate | 0.5-1.5% | 1.0% | Alternative to magnesium stearate |
| Sodium lauryl sulfate (SLS) | 1.0-5.0% | 2.0-3.0% | Reduces ejection force but not effective for punch sticking |
| Colloidal silicon dioxide | 0.1-0.5% | 0.2-0.3% | Often used in combination with other lubricants |
Implementation Guidelines:
- Start at lower end of range and increase if sticking persists
- Verify lubricant blending time (typically 3-5 minutes for magnesium stearate)
- Ensure lubricant particle size appropriate (200-100 mesh standard)
- Confirm uniform distribution through blending validation
Improper Lubricant Distribution:
Even with correct concentration, inadequate mixing prevents uniform lubricant coating on particle surfaces.
Solutions:
- Extend mixing time (but avoid over-mixing, which reduces dissolution)
- Verify blender functionality and mixing pattern
- Use appropriate mixing speed for lubricant type
- Consider pre-blending lubricant with small portion of formulation before final mix
- Implement in-process checks for lubricant distribution uniformity
Over-Lubrication Risks:
While increasing lubricant concentration often helps sticking, excessive amounts create new problems:
- Reduced tablet hardness and tensile strength
- Prolonged disintegration and dissolution times
- Potential interference with API release
- Soft, crumbly tablets
Balance sticking prevention against these negative effects by using minimum effective lubricant concentration.
Moisture and Hygroscopic Materials
Moisture content profoundly affects tablet sticking by creating capillary bridges between particles and punch surfaces.
Excessive Granule Moisture:
Insufficiently dried granules represent a primary sticking cause. Target moisture content varies by formulation but generally:
- Direct compression: <2% moisture
- Wet granulation formulations: 1-3% moisture post-drying
- Hygroscopic formulations: May require <1% moisture
Testing and Control:
- Implement loss-on-drying (LOD) testing at defined intervals
- Establish formulation-specific moisture specifications
- Validate drying equipment temperature uniformity
- Monitor drying time and endpoint criteria
- Conduct moisture testing before compression begins
Drying Protocol Optimization:
When moisture proves problematic:
- Extend drying time in 30-minute increments
- Increase drying temperature within API stability limits
- Improve air circulation in drying equipment
- Reduce granule bed depth for more uniform drying
- Consider fluid bed drying for better moisture removal
Hygroscopic Formulation Management:
Active pharmaceutical ingredients and excipients that absorb atmospheric moisture require special handling:
Hygroscopic APIs: Ibuprofen (slightly hygroscopic), certain salts, amorphous forms Hygroscopic excipients: Sorbitol, certain sugars, some cellulose derivatives
Strategies for hygroscopic formulations:
- Compress under controlled low-humidity conditions (30-40% RH maximum)
- Minimize granule exposure time before compression
- Store dried granulation in sealed, desiccated containers
- Consider using moisture barrier in formulation
- Modify granulation process to reduce hygroscopicity
- Implement environmental controls in compression area
Environmental humidity control proves essential—see Environmental Control Strategies section for specific protocols.
Granulation Characteristics
Beyond moisture content, granule physical properties significantly impact sticking propensity.
Granule Size Distribution:
Optimal granule size depends on formulation and tablet press specifications, but general principles apply:
Too fine granules (high fines content >20%):
- Increased surface area promotes adhesion
- Poor flow characteristics
- Greater sticking propensity
- Inconsistent fill weight
Too coarse granules:
- Segregation issues
- Variable content uniformity
- Potential for weak points in tablet
Optimization strategies:
- Target granule size distribution: 150-600 microns for most formulations
- Control fines through screening after drying
- Optimize granulation parameters to reduce fines generation
- Consider granule densification if size distribution problematic
Granule Strength:
Weak or overly soft granules fracture during compression, creating particles that adhere to punch faces rather than bonding into cohesive tablets.
Assessment methods:
- Friability testing of granules
- Visual/tactile assessment of granule integrity
- Compression force vs. tablet hardness profiling
Solutions for weak granules:
- Increase binder concentration (within limits)
- Optimize granulation technique (wetting, mixing time)
- Improve kneading during wet granulation
- Ensure adequate drying without over-drying (creates brittle granules)
- Consider alternate granulation method (e.g., wet vs. dry granulation)
Overly hard granules:
Conversely, excessively hard granules resist deformation during compression, potentially creating internal stress and sticking:
Solutions:
- Reduce binder concentration
- Decrease wet massing time
- Adjust granulation liquid addition rate
- Consider granule size reduction
API-Specific Challenges
Certain active pharmaceutical ingredients present inherent challenges for sticking prevention.
Low Melting Point APIs:
APIs with melting points below compression-generated temperatures (typically <70-80°C) create significant sticking risk through thermoplastic adhesion.
Common low melting point APIs:
- Ibuprofen (75-78°C)
- Certain waxes and lipids
- Some thermolabile compounds
Mitigation strategies:
- Reduce compression speed to minimize frictional heat
- Control compression room temperature (18-22°C recommended)
- Use cooling systems for particularly sensitive materials
- Select anti-stick coatings optimized for low melting point formulations
- Consider granulation methods that coat/protect API particles
- Add high-melting excipients to raise formulation softening point
Particle Size Effects:
API particle size and morphology influence sticking through several mechanisms:
Very fine particles (<10 microns):
- Increased surface area creates more adhesion points
- Poor flow characteristics
- Electrostatic issues
Irregular or needle-like particles:
- Mechanical interlocking with punch surfaces
- Inconsistent compression behavior
Solutions:
- Optimize API milling to target 20-100 micron range for most formulations
- Consider spray drying or other particle engineering approaches
- Use appropriate filler/diluent to dilute fine API
- Ensure adequate glidant addition
Surface Chemistry Considerations:
API surface properties (hydrophobicity, surface energy, charge) affect adhesion to tooling surfaces. While often fixed by molecular structure, formulation approaches can mitigate issues:
- Surfactants to modify surface interactions
- Coating technologies for API particles
- Excipient selection to complement API surface properties
- pH adjustment in granulation fluid (when applicable)
Tooling-Related Solutions

When formulation is established or cannot be changed, tooling modifications provide effective sticking solutions.
Punch Surface Specifications
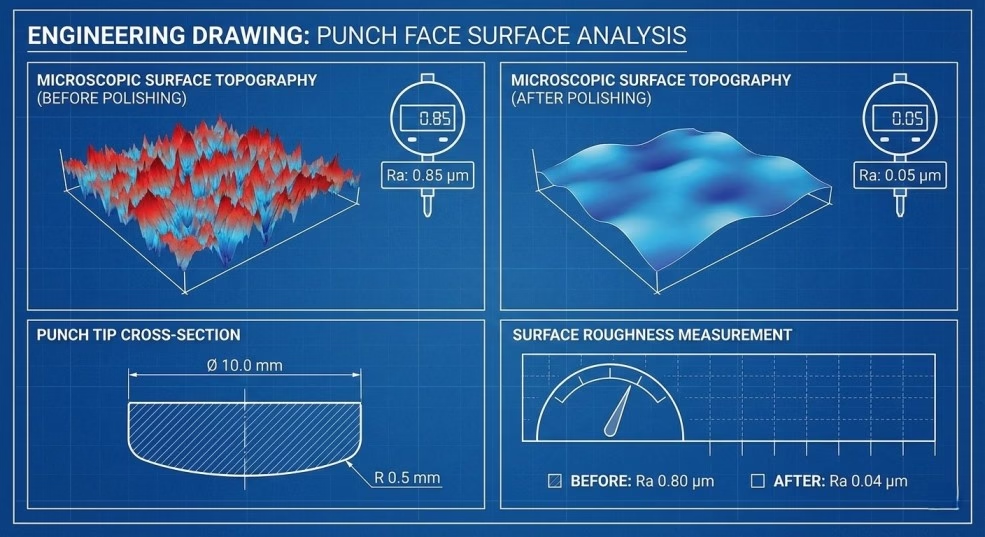
Punch face surface finish critically impacts sticking propensity through its influence on contact area and adhesion mechanisms.
Optimal Surface Roughness:
Surface roughness is quantified by Ra value (average roughness):
- Recommended Ra range: 0.2-0.4 micrometers for most applications
- Too smooth (<0.2 μm): Paradoxically increases sticking through larger true contact area
- Too rough (>0.4 μm): Creates mechanical interlocking points for particle adhesion
Surface Pattern Options:
Different surface finishes create varying performance characteristics:
Concentric pattern: Circular polishing marks radiating from center
- Advantages: Traditional finish, well-understood performance
- Best for: Standard formulations without specific sticking challenges
Crosshatch pattern: Intersecting linear polish marks
- Advantages: May improve material release for certain formulations
- Best for: Formulations showing directional sticking patterns
Random/multidirectional: No consistent pattern orientation
- Advantages: Minimizes directional effects
- Best for: Complex formulations with multiple sticky components
Inspection and Maintenance:
Regular surface inspection prevents gradual degradation leading to sticking:
Microscopy examination:
- Use optical microscopy (50-200x magnification) to identify:
- Micro-scratches from abrasive materials
- Coating wear or delamination
- Corrosion or chemical attack
- Material buildup in surface irregularities
Inspection frequency:
- New tooling: Before first use
- Routine production: Every 50,000-100,000 tablets (formulation-dependent)
- After aggressive cleaning: Verify no surface damage
- When sticking begins: Immediate inspection to assess contribution
Surface Damage Indicators Requiring Action:
| Observation | Likely Cause | Remedy |
|---|---|---|
| Visible scratches >2 microns | Abrasive formulation, improper handling | Re-polish or replace punches |
| Coating wear spots | Coating delamination, excessive use | Re-coat punches |
| Pitting or corrosion | Chemical attack, cleaning solution incompatibility | Replace punches, review cleaning protocols |
| Buildup in micro-scratches | Inadequate cleaning, surface too rough | Aggressive cleaning or re-polish |
| Uneven wear patterns | Turret alignment issues, inconsistent compression | Equipment maintenance, punch replacement |
Anti-Stick Coatings Comparison
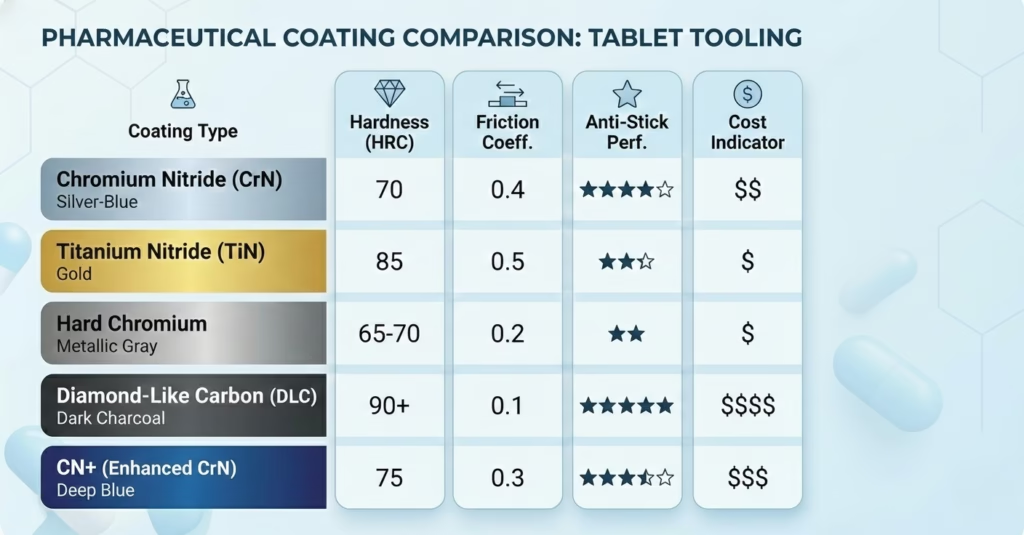
Surface coatings modify punch surface chemistry and topography to reduce adhesion. Multiple coating technologies exist, each with specific advantages.
Comprehensive Coating Comparison:
| Coating Type | Hardness (HV) | Coefficient of Friction | Corrosion Resistance | Anti-Stick Performance | Typical Cost | Best Applications |
|---|---|---|---|---|---|---|
| Chromium Nitride (CrN) | 1600-2000 | 0.4-0.5 | Excellent | Excellent | Moderate | General purpose anti-stick; hygroscopic formulations; first-line choice for sticky materials |
| Chromium Nitride Plus (CN+) | 1800-2200 | 0.3-0.4 | Excellent | Superior | Moderate-High | Extremely sticky formulations; complex embossing; proven severe sticking cases |
| Titanium Nitride (TiN) | 2000-2400 | 0.5-0.7 | Good | Good | Low-Moderate | Wear resistance priority; moderate sticking; cost-effective option |
| Hard Chromium (HC) | 800-1000 | 0.6-0.8 | Very Good | Moderate | Low | Standard applications; mild sticking; corrosion protection |
| Nickel Fluoropolymer | 400-600 | 0.1-0.2 | Good | Excellent | High | Extremely soft formulations; maximum release; limited wear resistance |
| Titanium Chromium Nitride (TiCrN) | 2000-2500 | 0.4-0.5 | Excellent | Very Good | Moderate | Multi-purpose; combines TiN and CrN benefits |
| Diamond-Like Carbon (DLC) | 2000-3000 | 0.1-0.15 | Excellent | Superior | High | Premium applications; effervescent tablets; maximum performance |
Selection Criteria:
Choose Chromium Nitride (CrN) when:
- Dealing with moderately sticky formulations
- Anti-stick performance is primary concern
- Hygroscopic or moisture-sensitive materials
- Budget allows moderate coating investment
- General-purpose solution needed
Choose Titanium Nitride (TiN) when:
- Wear resistance is priority over anti-stick
- Formulation has mild sticking tendency
- Cost control is important
- Abrasive formulations cause rapid punch wear
Choose Hard Chromium (HC) when:
- Standard formulation without significant sticking
- Corrosion protection primary need
- Budget-conscious approach required
- Establishing baseline before advanced coatings
Choose Advanced Coatings (CN+, DLC, Nickel Fluoropolymer) when:
- Severe sticking persists despite other interventions
- High-value product justifies premium coating
- Extremely difficult formulation characteristics
- Maximum performance required
Application Methods:
Physical Vapor Deposition (PVD):
- Low temperature process (<300°F) prevents dimensional changes
- Creates thin, uniform coatings (1-5 microns)
- Excellent adhesion to substrate
- Used for: TiN, CrN, TiCrN, DLC
- Benefits: Precise control, environmentally friendly, minimal part distortion
Electroplating:
- Chemical deposition process
- Can create thicker coatings (10-100 microns)
- Used for: Hard Chrome, Nickel
- Benefits: Cost-effective for some applications, well-established
Electron Beam (EB) Process:
- Specialized PVD variant
- Smoothest, most defect-free coatings
- Used for: Advanced CrN formulations (e.g., PharmaCote CN+)
- Benefits: Minimal droplet formation, superior surface quality
Coating Lifespan and Maintenance:
Expected coating durability varies significantly:
- TiN, CrN standard coatings: 50,000-200,000 tablets depending on formulation abrasiveness
- Advanced coatings (CN+, DLC): 100,000-500,000+ tablets
- Hard Chrome: 25,000-100,000 tablets
Factors affecting coating life:
- Formulation abrasiveness
- Compression force magnitude
- Cleaning method aggressiveness
- Coating quality and adhesion
- Underlying substrate quality
Coating Degradation Signs:
- Return of sticking in previously resolved situations
- Visible wear spots on punch faces
- Color changes indicating coating wear
- Increased friction during compression
Re-coating Considerations:
- Most coatings can be stripped and re-applied multiple times
- Substrate inspection essential before re-coating
- Cost-benefit vs. punch replacement analysis recommended
- Typically cost-effective to re-coat high-quality punch sets
Tablet Design Considerations
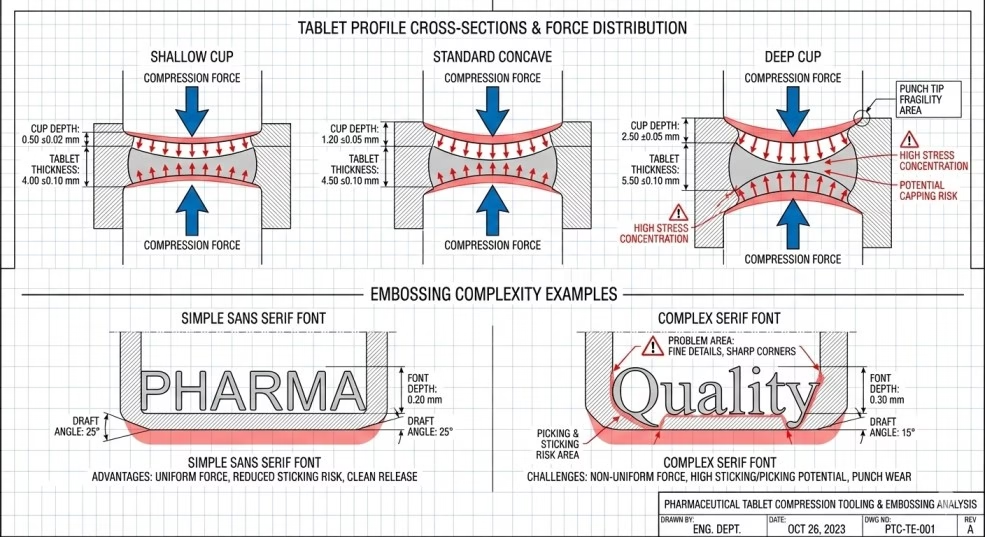
Tablet design—cup depth, profile, embossing—significantly influences sticking and picking propensity.
Cup Depth Impact:
Deeper cup profiles create larger punch-formulation contact area and may produce softer tablet cores prone to sticking.
Profile recommendations for sticky formulations:
- Avoid: Deep cup profiles (ball, deep pill)
- Prefer: Shallow cup, standard concave, or flat face with bevel
- Cup depth guidance: Generally <3mm depth for problematic formulations
Rationale: Shallow profiles:
- Reduce contact area between formulation and punch
- Create denser, harder tablet cores less prone to adhesion
- Provide more area for embossing without excessive cup depth
Embossing Design for Picking Prevention:
Complex embossing creates recessed areas where material readily adheres.
Font Selection:
| Font Style | Picking Risk | Recommended Use |
|---|---|---|
| Sans serif (e.g., Arial, Helvetica) | Low | First choice for all tablets; clean, simple characters minimize picking |
| Simple serif (e.g., Times Roman) | Moderate | Acceptable if brand requirements dictate; use larger sizes |
| Decorative/ornate serif | High | Avoid for sticky formulations; picking almost inevitable |
| Script or cursive | Very High | Avoid; extremely prone to picking |
Design Element Optimization:
Letter Height: Minimum 2-3mm for adequate visibility while maintaining manufacturability
Stroke Thickness: Avoid very thin strokes (<0.3mm) that create deep, narrow recesses
Character Spacing: Adequate spacing prevents bridging between letters where material accumulates
Blend Radii: Rounded transitions between embossed areas and cup surface reduce stress concentration and material trapping
Pre-Picking and Tapering Techniques:
Advanced engraving methods reduce picking in complex designs:
Pre-picking: Removing small “islands” (fully enclosed areas within letters like “O”, “R”, “P”) before production eliminates powder trapping
Tapering: Gradually angling the sides of embossed peninsulas (partially enclosed areas) facilitates powder release
Implementation: Specify these modifications during tooling design phase with experienced tool manufacturer
Compound Cup Configurations:
For tablets requiring both deep cups and complex embossing, compound cup designs provide solutions:
Concept: Two-radius cup profile allows:
- Shallower areas around embossing for better compression
- Deeper areas in non-embossed regions for desired tablet shape
Benefits:
- Optimized compression in embossed zones reduces picking
- Maintains desired tablet appearance
- Improves overall manufacturability
Application: Custom design for problematic tablets; consult tooling specialist
Tooling Maintenance Protocols
Systematic maintenance prevents gradual degradation that leads to sticking.
7-Step Maintenance Method:
1. Cleaning:
- Remove all tablet residue and powder immediately after use
- Use appropriate cleaning methods:
- Ultrasonic cleaning for routine maintenance
- Solvent cleaning when necessary (verify coating compatibility)
- Avoid abrasive materials that damage surfaces
- Cleaning frequency: After each batch for sticky formulations; less frequent for non-problematic materials
2. Assessment:
- Visual inspection under magnification (10-20x minimum)
- Identify damage, wear, or coating degradation
- Document findings for trend analysis
3. Repair:
- Address identified issues:
- Polish minor surface imperfections
- Re-coat worn coatings
- Replace damaged punches beyond repair
- Use qualified tooling service providers
4. Measurement:
- Verify critical dimensions maintained:
- Tip thickness
- Cup depth
- Overall length
- Barrel diameter
- Compare to original specifications
- Replace if out of tolerance
5. Polishing:
- Re-establish optimal surface finish when needed
- Use appropriate polishing compounds for coating type
- Verify Ra value after polishing
- Avoid excessive polishing that removes coating or alters dimensions
6. Lubrication:
- Apply appropriate lubricant to punch barrels (not faces)
- Use pharmaceutical-grade lubricants
- Prevents binding in turret guides
- Follow press manufacturer recommendations
7. Storage:
- Store in clean, dry, organized environment
- Use protective cases or racks preventing damage
- Control humidity to prevent corrosion
- Maintain inventory tracking system
Maintenance Frequency Recommendations:
| Formulation Characteristics | Inspection Interval | Deep Maintenance |
|---|---|---|
| Non-sticky, non-abrasive | Every 100,000 tablets | Every 500,000 tablets |
| Moderately sticky or abrasive | Every 50,000 tablets | Every 200,000 tablets |
| Highly sticky or very abrasive | Every 25,000 tablets | Every 100,000 tablets |
| Known problematic formulations | After each batch | Every 50,000 tablets |
Process Parameter Optimization

Process parameters offer rapid, low-cost adjustments that can significantly reduce sticking without formulation or tooling changes.
Compression Force Adjustment
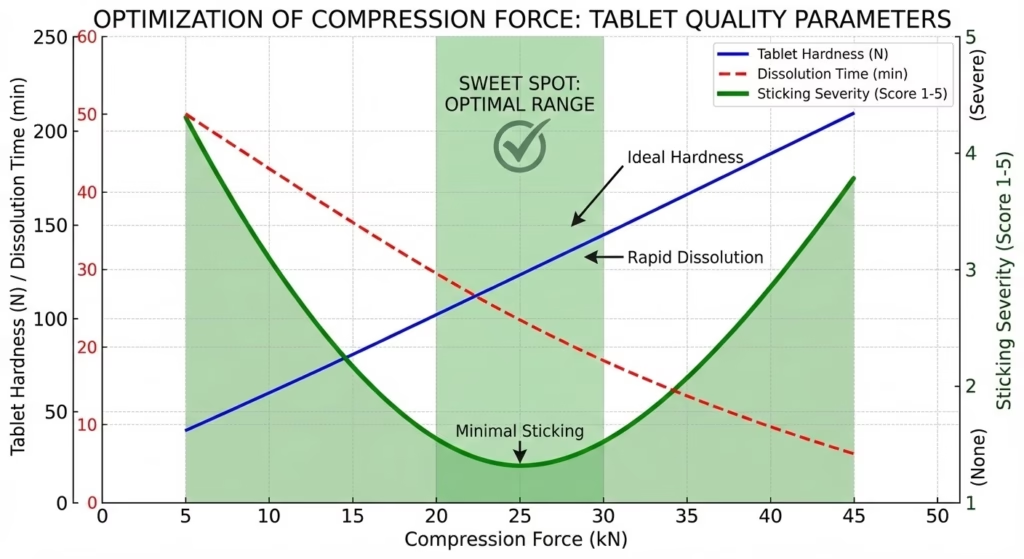
Compression force profoundly affects tablet sticking through its influence on compact density, heat generation, and ejection stress.
Insufficient Compression Force:
Too-little force creates weak tablets with poor interparticle bonding. During ejection, these weak cohesive forces allow material to separate and adhere to punch faces rather than staying with the tablet.
Indicators of insufficient force:
- Tablets soft, easily broken
- Low tablet hardness values
- Friability exceeds specifications
- Sticking accompanies general quality problems
Solution: Incrementally increase compression force:
- Increase by 1-2 kN increments for small tablets
- Increase by 2-5 kN increments for larger tablets
- Test tablet hardness, friability after each adjustment
- Stop increasing when target hardness achieved and sticking minimized
Excessive Compression Force:
Conversely, too-much force creates problems:
- Generates frictional heat that may soften thermoplastic components
- Creates excessive lateral forces against die walls
- May cause capping or lamination
- Can actually increase sticking for heat-sensitive formulations
Indicators of excessive force:
- Tablets very hard, potentially exceeding dissolution requirements
- Capping or lamination
- High ejection forces
- Sticking accompanied by temperature rise
Solution: Reduce compression force while maintaining quality:
- Decrease incrementally as above
- Monitor tablet hardness, dissolution
- Find minimum force providing required quality
- Balance sticking reduction against other quality attributes
Optimal Force Determination:
The “sweet spot” compression force:
- Provides required tablet hardness and friability
- Minimizes heat generation
- Maintains acceptable disintegration/dissolution
- Produces minimal sticking
Method for finding optimal force:
- Create compression force vs. tablet quality profile
- Plot hardness, friability, disintegration, sticking severity against force
- Identify force range meeting all specifications
- Select mid-point of acceptable range for robustness
Force Uniformity:
Inconsistent compression force across turret stations creates variable sticking:
Causes of force variation:
- Uneven punch lengths
- Turret wear or alignment issues
- Compression roll wear
- Pre-compression adjustment problems
Solutions:
- Verify all punches within length tolerance (typically ±0.025mm)
- Conduct press qualification/calibration
- Replace worn components
- Balance pre-compression and main compression forces
Compression Speed and Dwell Time
The time formulation spends under compression (dwell time) significantly affects sticking for certain materials.
Dwell Time Fundamentals:
Dwell time = time formulation remains at or near maximum compression force
Calculated as: Dwell time (ms) ≈ (Contact arc angle / Rotation speed)
For rotary tablet presses:
- Higher speeds = shorter dwell time
- Larger compression roll diameter = longer dwell time at same speed
Dwell Sensitivity:
Formulations with viscoelastic or plastic properties exhibit dwell time sensitivity:
Short dwell times:
- Less time for stress relaxation
- May produce weaker tablets prone to sticking
- Beneficial for heat-sensitive materials (less frictional heating)
Long dwell times:
- More complete consolidation
- Potential for greater heat generation
- Risk of material adhesion from prolonged contact
Speed Adjustment Strategy:
For most sticky formulations, reducing speed helps:
Benefits of slower compression:
- Reduced frictional heat generation
- More controlled ejection
- Better stress distribution
- Lower mechanical shock
Implementation:
- Reduce speed by 10-20% increments
- Monitor impact on sticking
- Balance against production throughput needs
- Document optimal speed for each formulation
Typical speed ranges:
- Single punch presses: 10-40 tablets/minute
- Small rotary presses: 5,000-30,000 tablets/hour
- High-speed rotary presses: 100,000-400,000+ tablets/hour
Trade-offs:
- Slower speed reduces throughput
- May require additional press time or equipment
- Economic analysis: sticking-related losses vs. speed reduction costs
When to Increase Speed:
Occasionally, certain formulations benefit from faster compression (shorter dwell time):
- Extremely time-dependent materials that worsen with prolonged contact
- Heat-sensitive materials where brief contact minimizes temperature rise
Test both directions when troubleshooting to identify optimal speed.
Pre-Compression Strategies
Pre-compression (applying partial force before main compression) can reduce sticking for specific formulations.
Pre-Compression Benefits:
- Gradual particle rearrangement before full compression
- Air evacuation reducing entrapment
- Stress distribution improvement
- Potential sticking reduction through modified compression profile
Pre-Compression Force Ratios:
Typical pre-compression force: 10-30% of main compression force
Adjustment approach:
- Start with 15-20% pre-compression ratio
- Adjust incrementally
- Monitor sticking and tablet quality
- Find optimal ratio for formulation
When Pre-Compression Helps Sticking:
- Formulations with poor air evacuation
- Fluffy, low-density granules
- Materials requiring gradual consolidation
- High-dose formulations with minimal excipients
When Pre-Compression May Worsen Sticking:
- Already dense formulations
- Materials sensitive to extended compression time
- Very heat-sensitive formulations
Implementation:
- Available on most modern rotary presses
- Adjust via press control system
- Validate effect through systematic testing
Environmental Control Strategies

Environmental factors—particularly temperature and humidity—critically impact tablet sticking, especially for sensitive formulations.
Temperature Management
Compression generates frictional heat, and ambient temperature affects thermoplastic formulation behavior.
Compression Room Temperature Recommendations:
Standard formulations: 20-25°C (68-77°F)
Heat-sensitive/low melting point formulations: 18-22°C (64-72°F)
Very sensitive materials (e.g., ibuprofen): 16-20°C (61-68°F)
Temperature Control Implementation:
HVAC System Design:
- Dedicated air handling for compression areas
- Redundant cooling capacity
- Temperature monitoring and alarming
- Seasonal adjustment capability
Monitoring Requirements:
- Continuous temperature recording in compression area
- Setpoint: ±2°C tolerance
- Data logging for correlation with sticking incidents
- Alert systems for out-of-range conditions
Seasonal Variation Management:
Summer months create special challenges:
- Increase HVAC capacity or adjust production schedules
- Consider night/early morning production for heat-sensitive products
- Monitor and document temperature effects
- Implement contingency protocols for temperature excursions
Equipment Heat Generation:
Tablet press operation generates significant heat:
Mitigation strategies:
- Adequate ventilation around press
- Equipment maintenance to minimize bearing friction
- Scheduled production breaks for cooling (if needed)
- Press enclosure venting systems
Low Melting Point API Specific Protocols:
For APIs like ibuprofen (MP 75-78°C), granulation temperature approaches critical as compression friction adds heat:
Comprehensive temperature control:
- Cool compression room to lower limit (18°C)
- Store granulation in temperature-controlled area before compression
- Minimize granulation exposure before use
- Reduce compression speed (less frictional heat)
- Implement press cooling if available
- Consider granule pre-cooling (refrigerated storage, controlled warming before use)
- Use tooling coatings optimized for thermoplastic materials
Humidity Control

Relative humidity dramatically affects sticking through moisture adsorption and capillary bridge formation.
Target Humidity Ranges by Formulation Type:
| Formulation Characteristics | Recommended RH | Maximum RH |
|---|---|---|
| Non-hygroscopic, standard formulations | 40-50% | 60% |
| Slightly hygroscopic materials | 35-45% | 50% |
| Moderately hygroscopic formulations | 30-40% | 45% |
| Highly hygroscopic materials | 25-35% | 40% |
| Extreme hygroscopic sensitivity | 20-30% | 35% |
Humidity Control Systems:
Dehumidification Options:
Refrigerant dehumidifiers:
- Cost-effective for moderate control
- Suitable for 40-60% RH targets
- Limited effectiveness below 40% RH
Desiccant dehumidifiers:
- Effective for low humidity requirements (<40% RH)
- Higher operating costs
- Necessary for highly hygroscopic formulations
Combined systems:
- Refrigerant for bulk moisture removal
- Desiccant for fine control and low humidity
- Most efficient for pharmaceutical applications
Implementation Best Practices:
Room design:
- Vapor barriers in construction
- Sealed penetrations and doorways
- Airlocks for material transfer
- Positive pressure relative to adjacent areas (prevents humid air infiltration)
Monitoring and Control:
- Continuous RH measurement at multiple points
- Data logging with correlation to production
- Setpoint control: ±5% RH tolerance
- Alarming for out-of-specification conditions
Seasonal Challenges:
High Humidity Seasons (summer in humid climates):
- Increased dehumidification demand
- More frequent system maintenance
- Production scheduling considerations
- Enhanced monitoring
Low Humidity Seasons (winter, dry climates):
- Static electricity concerns
- Potential for over-drying
- System modulation to prevent excessive drying
Hygroscopic Formulation Specific Protocols:
For materials like sorbitol, certain sugar-based formulations:
- Storage Control:
- Maintain dried granulation in sealed containers with desiccant
- Minimize exposure time before compression
- Implement first-in-first-out inventory management
- Compression Area Protocol:
- Dedicated low-humidity compression suite (if multiple products manufactured)
- Personnel training on humidity-sensitive handling
- Minimal container opening time
- Rapid material transfer to press hopper
- Process Timing:
- Compress immediately after granule preparation when possible
- Avoid overnight storage of prepared granulation
- Document time-out-of-container for correlation with sticking
- Backup Plans:
- Alternative compression location if primary area humidity control fails
- Re-drying protocols for granulation exposed to excessive humidity
- Humidity excursion investigation and material disposition procedures
Capillary Bridge Formation Prevention:
Humidity creates thin moisture layers on particle and tooling surfaces, forming capillary bridges that dramatically increase adhesion.
Mechanism: Water adsorbs onto punch faces and granule surfaces, creating liquid bridges under compression that resist tablet separation during ejection.
Prevention approaches:
- Maintain humidity below formulation-specific threshold
- Use hydrophobic coatings on punches (reduce moisture adsorption)
- Optimize lubricant selection (hydrophobic lubricants provide moisture barrier)
- Environmental control as primary defense
Documentation Requirements:
Environmental monitoring records should include:
- Continuous temperature and RH data
- Excursion documentation and investigation
- Correlation with sticking incidents
- Seasonal pattern identification
- System maintenance records
- Calibration of monitoring equipment
This documentation supports:
- Root cause analysis when sticking occurs
- Validation of environmental control effectiveness
- Regulatory compliance (cGMP requirements)
- Continuous improvement initiatives
Advanced Solutions and Technologies
For persistent sticking problems or high-value products, advanced technologies provide sophisticated solutions.

Predictive Modeling (TSAR and Similar Tools)
The Tabletting Science Anti-Stick Research (TSAR) program, developed by I Holland in collaboration with University of Nottingham, represents a breakthrough in predicting sticking before production.
How Predictive Models Work:
TSAR integrates multiple parameters:
- Formulation surface chemistry
- Particle size distribution
- Granule mechanical properties (elastic vs. plastic behavior)
- Environmental conditions (temperature, humidity)
- Punch coating type and surface characteristics
- Compression parameters
Analytical Techniques Used:
Atomic Force Microscopy (AFM):
- Maps adhesive forces across punch face surfaces
- Identifies localized high-adhesion zones
- Measures adhesion at molecular scale
X-ray Photoelectron Spectroscopy (XPS):
- Analyzes surface chemical composition
- Identifies reactive species
- Characterizes coating chemistry
Time-of-Flight Secondary Ion Mass Spectrometry (TOF-SIMS):
- Visualizes chemical distribution on surfaces
- Detects trace contaminants
- Maps coating uniformity
Principle Component Analysis (PCA):
- Statistical correlation of multiple variables
- Identifies primary factors driving sticking
- Predicts coating performance for specific formulations
Benefits of Predictive Screening:
Risk Reduction:
- Identify sticking risk before scale-up
- Test coating options in laboratory vs. production
- Avoid costly production failures
Coating Selection:
- Scientifically select optimal coating for formulation
- Avoid trial-and-error testing
- Reduce tooling costs through first-time-right selection
Time Savings:
- Eliminate in-field testing (weeks → days)
- Reduce development timeline
- Accelerate troubleshooting when problems arise
Cost Benefits:
- Prevent production downtime
- Optimize coating investment
- Reduce batch losses
Accessibility:
TSAR and similar programs typically offered by:
- Tooling manufacturers (I Holland, others)
- Contract research organizations
- Academic institutions with pharmaceutical technology programs
Implementation approach:
- Submit formulation composition and problem description
- Provide granule samples for testing
- Receive coating recommendation based on predictive analysis
- Implement recommended coating
- Validate performance in production
Gravimetric Screening Methods

Pfizer developed a quantitative screening tool enabling early assessment of sticking propensity during formulation development.
Method Overview:
Custom punch design: Removable punch tip allows gravimetric measurement of adhered powder after compression cycles.
Procedure:
- Compress defined number of tablets using test formulation
- Remove punch tip
- Weigh adhered material
- Calculate sticking index: mg material adhered per 100 tablets
Sticking Severity Classification:
| Sticking Index (mg/100 tablets) | Severity | Action |
|---|---|---|
| <5 mg | Minimal | Proceed; routine monitoring |
| 5-15 mg | Mild | Monitor closely; minor optimization recommended |
| 15-30 mg | Moderate | Optimize formulation/process before scale-up |
| 30-50 mg | Severe | Significant intervention required |
| >50 mg | Critical | Reformulation likely necessary |
Applications:
Formulation Development:
- Screen multiple binder options
- Optimize lubricant type and concentration
- Compare granulation methods
- Assess API particle size effects
- Rank multiple formulation variants
Scale-Up Risk Assessment:
- Predict production sticking likelihood
- Plan tooling coating requirements
- Establish cleaning frequency
- Set inspection intervals
Root Cause Diagnosis:
- Systematically test individual formulation components
- Isolate contributing factors
- Validate solution effectiveness
Manufacturing Support:
- Troubleshoot unexpected sticking
- Compare different material lots
- Qualify suppliers or processes
Implementation Requirements:
Equipment:
- Custom tableting punch with removable tip
- Analytical balance (±0.1 mg precision)
- Small-scale tablet press or compaction simulator
Materials:
- Small quantities of test formulation (10-50g)
- Standard test conditions (defined force, speed)
Personnel:
- Training in standardized method
- Consistent technique for reproducibility
Benefits:
- Quantitative assessment (vs. subjective visual inspection)
- Early warning during development
- Comparative evaluation of multiple options
- Minimal material requirements (development quantities)
- Fast results (hours vs. days/weeks)
Limitations:
- Requires specialized equipment
- May not perfectly predict large-scale behavior
- Formulation and scale-dependent correlation
- Validation needed for each new formulation family
Surface Modification Technologies
Advanced surface treatments beyond traditional coatings offer additional sticking solutions.
Plasma Treatment:
Process: Expose punch surfaces to ionized gas (plasma) under controlled conditions.
Effects:
- Modifies surface energy and chemistry
- Creates hydrophobic or hydrophilic surfaces as needed
- Can improve coating adhesion
- Removes contaminants
Applications:
- Pre-treatment before coating application
- Direct surface modification for specific formulations
- Enhancing coating performance
Textured Surfaces:
Concept: Deliberately create controlled micro-texture patterns on punch faces.
Methods:
- Laser ablation for precise pattern creation
- Chemical etching
- Mechanical texturing with controlled parameters
Benefits:
- Reduced true contact area (paradoxically can reduce adhesion)
- Improved coating adhesion
- Customizable for specific formulation needs
Challenges:
- Difficult to clean textured surfaces
- May trap material requiring aggressive cleaning
- Pattern design requires expertise
Best for: Specialized applications where conventional approaches insufficient
Emerging Coating Technologies:
Nano-layered coatings:
- Alternating layers of different materials at nanometer scale
- Combines benefits of multiple coating types
- Superior mechanical properties
- Currently research/development stage for pharmaceutical applications
Adaptive/smart coatings:
- Respond to environmental conditions
- Change properties based on temperature, humidity
- Experimental stage; potential future solutions
Self-lubricating coatings:
- Incorporate solid lubricants within coating matrix
- Continuously release lubricating material
- Extended performance without maintenance
Solution Selection Framework
With multiple intervention options available, systematic selection prevents wasted effort and resources.
Quick Wins vs. Long-Term Solutions
Prioritize interventions by implementation speed and resource requirements.
Immediate Adjustments (Hours to Days):
Process parameter optimization:
- Reduce compression speed: 1-4 hours to test and implement
- Adjust compression force: 1-2 hours
- Verify environmental conditions: Immediate
- Enhance cleaning frequency: Immediate
Cost: Minimal (primarily labor time) Effectiveness: 20-40% of sticking problems resolved through process optimization alone
Short-Term Improvements (Days to Weeks):
Formulation adjustments within specifications:
- Increase lubricant concentration: 1-3 days (mixing, testing)
- Extend drying time: 1-2 days
- Adjust mixing time: 1 day
Tooling maintenance:
- Deep cleaning and polishing: 1-3 days
- Surface inspection and assessment: 1 day
Environmental control enhancement:
- HVAC adjustment: Immediate to 1 week
- Humidity control optimization: 1-2 weeks
Cost: Low to moderate ($500-$5,000) Effectiveness: 30-50% of problems resolved with short-term adjustments
Long-Term Investments (Weeks to Months):
Tooling coating:
- Coating application: 2-4 weeks (remove, ship, coat, return, validate)
- Cost: $50-$200 per punch depending on coating type
Reformulation:
- Formulation development: 4-12 weeks
- Stability testing: 3-6 months
- Regulatory considerations: Variable (marketed products)
- Cost: $10,000-$100,000+ depending on extent
Equipment upgrade:
- New tablet press or compression simulator: Months
- Cost: $50,000-$500,000+
Cost: Significant ($5,000-$100,000+) Effectiveness: 80-95% of problems solvable with comprehensive approach
Cost-Benefit Analysis
Decision Matrix:
| Solution Category | Typical Cost | Implementation Time | Expected Effectiveness | Best Use Case |
|---|---|---|---|---|
| Process parameter adjustment | <$1,000 | Hours-Days | 20-40% | First-line intervention; process-related root cause |
| Environmental control | $1,000-$10,000 | Days-Weeks | 30-50% | Humidity/temperature-sensitive formulations |
| Formulation optimization (minor) | $2,000-$10,000 | Days-Weeks | 40-60% | Formulation-related root cause; within spec limits |
| Tooling maintenance/polishing | $500-$2,000 | Days | 20-40% | Tooling wear identified; surface damage |
| Tooling coating (standard) | $3,000-$10,000 | 2-4 Weeks | 50-70% | Tooling-related cause; sticky formulation established |
| Tooling coating (advanced) | $5,000-$15,000 | 2-4 Weeks | 60-80% | Severe sticking; premium coating justified |
| Reformulation (major) | $20,000-$100,000+ | 3-6 Months+ | 70-90% | Formulation fundamentally problematic; development stage |
| Predictive screening (TSAR) | $5,000-$15,000 | 1-2 Weeks | 60-80% | High-value product; coating selection critical |
ROI Calculation Approach:
Step 1: Quantify Current Sticking Cost
- Production downtime hours/week: ____ × $____ /hour = $____
- Batch rejection rate: ____ % × $____ /batch × ____ batches/year = $____
- Extra cleaning/maintenance: ____ hours/week × $____ /hour = $____
- Annual sticking cost: $____
Step 2: Estimate Solution Cost
- One-time implementation cost: $____
- Ongoing costs (if any): $____ /year
- Total first-year cost: $____
Step 3: Estimate Effectiveness
- Expected reduction in sticking incidents: ____ %
- Annual savings: Current cost × Effectiveness % = $____
Step 4: Calculate Payback
- Payback period: Solution cost ÷ Annual savings = ____ months
Decision Rule:
- Payback <3 months: Implement immediately
- Payback 3-6 months: Strong case; implement unless major constraints
- Payback 6-12 months: Evaluate against alternatives; implement if best option
- Payback >12 months: Reconsider; look for more cost-effective alternatives
When to Reformulate vs. When to Adjust Process/Tooling
This critical decision significantly impacts timeline and cost.
Reformulate When:
Indicators for reformulation:
- Formulation fundamentally problematic (inherent API characteristics)
- Multiple batches show persistent sticking despite all other interventions
- Development stage (flexibility still exists)
- Quality attributes compromised by current formulation
- Economic analysis shows reformulation cost-effective vs. ongoing issues
- Scale-up anticipated and current formulation high-risk
Reformulation advantages:
- Addresses root cause permanently
- May improve other quality attributes simultaneously
- Eliminates ongoing process complications
- Sustainable long-term solution
Reformulation challenges:
- Time-intensive (months)
- Expensive development work
- Stability studies required
- Regulatory implications for marketed products (variations, supplements, new filing)
- Risk of creating new problems
Adjust Process/Tooling When:
Indicators for process/tooling approach:
- Formulation validated and stable
- Marketed product (regulatory change burden)
- Sticking manageable with process control
- Cost-benefit favors tooling investment vs. reformulation
- Timeline constraints prevent reformulation
- Multiple products use similar formulation (tooling solution benefits multiple products)
Process/tooling advantages:
- Faster implementation (weeks vs. months)
- Lower cost for single product
- Minimal regulatory impact
- No stability re-validation required
- Reversible if ineffective
Process/tooling challenges:
- May not address fundamental root cause
- Ongoing vigilance required
- Process window may narrow
- Equipment-specific (less portable across sites)
Decision Framework:
Question 1: Is this a new product in development?
- Yes: Strong preference for reformulation; establish robust formulation upfront
- No: Proceed to Question 2
Question 2: Is the formulation validated/marketed with stability data?
- Yes: Strong preference for process/tooling unless severe problem
- No: Reformulation option more viable
Question 3: Have process and tooling optimizations been exhausted?
- Yes: Reformulation may be necessary
- No: Continue optimization before reformulation
Question 4: Does economic analysis favor reformulation?
- Calculate 5-year total cost of ownership for each approach
- Consider: Development costs, ongoing process complexity costs, tooling costs, scale-up risk, regulatory costs
Question 5: What is the timeline urgency?
- Immediate need (<3 months): Process/tooling only option
- Moderate timeline (3-6 months): Either approach feasible
- Long timeline (>6 months): Reformulation viable if indicated
Hybrid Approach:
Often optimal strategy combines approaches:
- Immediate: Process optimization and environmental control (stabilize production)
- Short-term: Tooling coating (improve robustness)
- Long-term: Formulation optimization for next product iteration or major revision
This staged approach maintains production while working toward optimal solution.
Case Studies: Real-World Problem Solving
Real-world examples demonstrate how systematic approaches yield measurable results.
Case Study 1: Coating Solution for Antiepileptic Drug

Company: Novartis (Italian division)
Problem Description:
Film-coated antiepileptic drug tablet experiencing severe sticking and picking during production. Press required stopping multiple times per batch for punch cleaning and maintenance, creating significant production downtime and reduced output.
Product characteristics:
- Complex embossed tablet design
- Film-coated formulation
- Required high compression forces
- Established marketed product (reformulation not viable option)
Diagnostic Approach:
Initial attempts (unsuccessful):
- Tried multiple standard coatings: Hard Chromium (HC), Chromium Nitride (CrN), Titanium Nitride (TN)
- Results unsatisfactory; sticking persisted
Systematic evaluation:
- Engaged I Holland tooling specialists
- Submitted sample tools for microscopic analysis
- Applied TSAR≈Predict (Tabletting Science Anti-Stick Research) predictive model
- Analyzed tablet design parameters
TSAR Analysis:
Model integrated:
- Formulation surface chemistry
- Environmental conditions (temperature, humidity)
- Granule size and elastic/plastic properties
- Multiple coating options
- Tablet profile and embossing design
Tablet design review identified:
- Deep cup profile creating softer tablet core (contributing to sticking)
- Complex embossing with multiple characters (picking risk)
- Need for optimized profile with shallower cup
Solution Implementation:
Two-pronged approach:
1. Tablet Design Modification:
- Created new tablet profile with reduced cup depth
- Implemented anti-picking features in embossing
- Optimized engraving to reduce material trapping
2. Advanced Coating Application:
- Selected PharmaCote CN+ (Chromium Nitride Plus) based on TSAR prediction
- Applied using electron beam PVD process (smoothest, most defect-free method)
- Coated both upper and lower punches
Results:
Dramatic production improvement:
- Production increase: 25% (5 batches in 5 days vs. 4 batches in 6 days previously)
- Downtime elimination: No production stops required for punch cleaning
- Quality improvement: Consistent tablet appearance, no picking defects
- Cost savings: One full day production time saved per week
Before implementation:
- 4 batches produced during 6-day work week
- Multiple production stops for cleaning
- Extra maintenance time required
- Inconsistent tablet quality
After implementation:
- 5 batches produced during 5-day work week
- Zero unscheduled stops
- Minimal maintenance requirements
- Consistent high-quality output
Outcome:
Novartis expanded use of this systematic approach to additional products experiencing sticking issues.
Key Success Factors:
- Used predictive modeling rather than trial-and-error
- Addressed multiple factors (design + coating) simultaneously
- Selected optimal coating through scientific analysis
- Implemented proven advanced coating technology
Case Study 2: Formulation Adjustment for Low-Melting API

Company: [Generic pharmaceutical manufacturer]
Problem Description:
Ibuprofen 200mg tablet experiencing severe sticking during compression, particularly in summer months. Material adhered to punch faces requiring cleaning every 30-60 minutes. Production significantly slowed; batch completion time doubled.
Product characteristics:
- Ibuprofen API (melting point 75-78°C)
- Direct compression formulation
- Round standard concave tablet
- High-volume product (critical to production schedule)
Diagnostic Approach:
Root cause analysis identified:
Primary factor: Thermoplastic adhesion
- Ibuprofen’s low melting point
- Compression friction generating heat
- API approaching softening point during compression
- Thermoplastic flow creating adhesion to punch faces
Contributing factors:
- Ambient temperature: 24-26°C (summer production)
- Compression speed: High (200,000+ tablets/hour for volume)
- Lubricant: 0.5% magnesium stearate (lower end of range)
- Environmental control: Moderate (not optimized for heat-sensitive materials)
Solution Implementation:
Multi-factorial approach (avoided reformulation):
1. Environmental Control:
- Reduced compression room temperature to 18-20°C
- Implemented enhanced monitoring and control (±1°C tolerance)
- Scheduled production during cooler hours when possible
2. Process Optimization:
- Reduced compression speed from 200,000 to 150,000 tablets/hour (25% reduction)
- Optimized compression force (reduced to minimum providing required hardness)
- Implemented more frequent short breaks allowing press cooling
3. Formulation Adjustment (Minor, Within Specifications):
- Increased magnesium stearate from 0.5% to 0.75%
- Extended lubricant mixing time from 3 to 5 minutes
- Verified lubricant particle size specification
4. Tooling Enhancement:
- Applied Chromium Nitride (CrN) coating to all punches
- Selected coating optimized for thermoplastic materials
- Verified punch surface finish specification
Results:
Sticking reduction: >90%
- Cleaning frequency: Every 30-60 minutes → Every 8-12 hours
- Production speed restored to 175,000 tablets/hour (compromise between original speed and sticking prevention)
- Summer production issues eliminated
- Quality maintained: Hardness, dissolution, appearance all within specifications
Economic impact:
- Downtime reduction: ~4 hours/batch saved
- Throughput optimization: Net improvement despite modest speed reduction
- Coating investment: ~$8,000 one-time cost
- Payback period: <2 months based on downtime savings
Key Success Factors:
- Recognized thermoplastic mechanism specific to low-melting API
- Implemented complementary solutions across multiple categories
- Avoided reformulation through systematic optimization
- Balanced speed reduction against overall productivity
Case Study 3: Environmental Control for Hygroscopic Formulation

Company: [Contract manufacturer]
Problem Description:
Extended-release tablet containing hygroscopic API and excipients experiencing inconsistent sticking—severe during humid weather, minimal during dry conditions. Problem unpredictable, causing production planning difficulties.
Product characteristics:
- Hygroscopic API (absorbs atmospheric moisture readily)
- Sorbitol as major excipient (also hygroscopic)
- Complex controlled-release formulation
- Wet granulation process
- Customer product (reformulation not manufacturer’s option)
Diagnostic Approach:
Pattern analysis revealed:
- Sticking severity correlated directly with ambient humidity
- Worse in summer/rainy periods
- Improved dramatically in winter/dry periods
- Same formulation, tooling, process parameters
Root cause identified:
- Moisture absorption during granule storage and handling
- Capillary bridge formation between hygroscopic materials and tooling
- Even well-dried granulation rapidly absorbed atmospheric moisture
- Critical threshold: ~45% relative humidity
Solution Implementation:
Comprehensive humidity control program:
1. Dedicated Compression Suite:
- Converted existing room to controlled low-humidity environment
- Installed desiccant dehumidification system (capable of 25-35% RH)
- Implemented positive pressure with filtered air
- Created airlock entry for material transfer
2. Material Handling Protocols:
- Stored dried granulation in sealed containers with desiccant packs
- Implemented maximum 2-hour out-of-container time limit
- Created SOP for container opening/closing procedures
- Used humidity indicator cards in storage containers
3. Process Modifications:
- Transferred granulation to compression room 24 hours before use (equilibrate to controlled environment)
- Minimized hopper exposure (covered when not actively filling)
- Implemented continuous RH monitoring with data logging
- Created environmental excursion investigation procedure
4. Seasonal Scheduling:
- Prioritized this product during naturally drier seasons when possible
- Maintained capability for year-round production via controlled environment
Results:
Sticking consistency: Achieved
- Humidity-related sticking eliminated
- Reproducible performance regardless of season
- Reduced process variability
- Improved production planning reliability
Economic analysis:
- Dehumidification system: $35,000 capital investment
- Room modification: $15,000
- Operating costs: ~$3,000/year (energy, maintenance)
- Total first-year cost: $53,000
Benefits:
- Eliminated 15-20 days/year of severely impaired production
- Value of improved productivity: ~$120,000/year
- Payback: ~5 months
- Enabled reliable scheduling (value difficult to quantify but significant)
Additional benefit: Controlled suite now available for other humidity-sensitive products, spreading investment value across multiple formulations.
Key Success Factors:
- Recognized environmental pattern through data analysis
- Implemented comprehensive control (not partial measures)
- Combined environmental control with handling protocols
- Created sustainable solution for ongoing production
Prevention Strategies During Development

Preventing sticking during formulation and process development is far more efficient than troubleshooting during production.
Formulation Design Principles
Lubricant Selection Early:
Build sticking prevention into formulation from initial development:
Screening approach:
- Test multiple lubricant types in early formulation trials
- Evaluate not just functionality but sticking propensity
- Consider using screening methods (gravimetric assessment)
- Select optimal lubricant before finalizing formulation
Lubricant considerations:
- For sticky formulations: Consider sodium stearyl fumarate or combination approaches
- For standard formulations: Magnesium stearate typically effective
- For moisture-sensitive: Hydrophobic lubricants preferred
Moisture Sensitivity Screening:
Assess hygroscopicity early in development:
Testing protocol:
- Expose formulation to controlled humidity conditions (40%, 60%, 75% RH)
- Monitor moisture uptake over time
- Compress samples after humidity exposure
- Assess sticking tendency
Decision points:
- Minimal moisture uptake (<1% at 60% RH): Standard environmental control adequate
- Moderate uptake (1-3% at 60% RH): Enhanced environmental control needed
- Significant uptake (>3% at 60% RH): Reformulation or stringent humidity control required
Granulation Method Selection:
Different granulation approaches create different sticking risks:
Wet granulation:
- Benefits: Strong granules, good content uniformity
- Sticking considerations: Moisture control critical, drying endpoint validation essential
- Best for: APIs requiring significant dilution, complex formulations
Dry granulation:
- Benefits: No moisture addition, simpler process
- Sticking considerations: May produce harder granules, potential for fine generation
- Best for: Moisture-sensitive APIs, direct compression unsuitable
Direct compression:
- Benefits: Simplest process, no moisture or heat
- Sticking considerations: Requires excellent lubricant distribution, API must have good compression properties
- Best for: APIs with favorable compression characteristics, simple formulations
Selection criteria should include sticking risk assessment alongside traditional factors (API stability, manufacturing cost, etc.)
Tooling Specification Best Practices
Design for Manufacturability:
Tablet design significantly impacts manufacturability; involve tooling specialists early:
Early consultation benefits:
- Identify potential sticking/picking issues before tooling fabrication
- Optimize embossing design for manufacturability
- Select appropriate profile and cup depth
- Plan coating requirements upfront
Design decisions impacting sticking:
Profile selection:
- Preference order for sticky formulations: Flat face with bevel > Shallow concave > Standard concave > Deep cup > Ball
- Balance aesthetic requirements against manufacturing robustness
Embossing complexity:
- Lower complexity designs for sticky formulations
- Use sans serif fonts when brand allows
- Minimize character count
- Adequate spacing between characters
- Appropriate letter height (>2-3mm)
Coating Selection During Design:
Specify coating proactively rather than reactively:
Decision framework:
Specify coating from beginning when:
- Known sticky API (low melting point, hygroscopic, etc.)
- Similar formulations historically problematic
- Complex embossing design
- Development timeline critical (prevent delays)
- High-value product (justify coating investment)
Standard tooling initially, coating if needed when:
- Standard formulation with good properties
- Simple design
- Development budget constrained
- Time available for iterative optimization
Coating specification approach:
- Discuss formulation characteristics with tooling manufacturer
- Consider predictive screening if available (TSAR)
- Select coating based on formulation needs, not just cost
- Specify coating in initial tooling order (avoid delay of retrofitting)
Scale-Up Considerations
Technology transfer from development to production frequently reveals sticking issues.
Common Sticking Issues During Scale-Up:
Equipment differences:
- Different tablet press models (turret size, compression characteristics)
- Different environmental control capabilities
- Higher production speeds
- Longer compression runs
Process scale differences:
- Granulation equipment scale-up effects
- Mixing scale differences affecting lubricant distribution
- Drying uniformity in larger batches
Material variability:
- Commercial-scale raw material lots vs. development quantities
- Supplier changes during scale-up
Pilot-to-Production Transition Planning:
Scale-up protocol should include:
1. Compression Simulation:
- Use compaction simulator to predict commercial press behavior
- Test at target production speed and force
- Assess sticking propensity before full-scale batches
2. Environmental Assessment:
- Verify production facility environmental control capabilities
- Match or exceed development conditions
- Plan enhancements if needed before transfer
3. Tooling Strategy:
- Fabricate production tooling identical to successful development tooling
- Consider coating production tooling even if development tooling uncoated (higher volume, longer runs)
- Order spare sets for rapid replacement if issues arise
4. Validation Batches:
- Plan small-scale production batches before full commercial runs
- Monitor sticking carefully
- Adjust parameters before high-volume production
5. Process Parameter Documentation:
- Transfer complete understanding of process window
- Document critical parameters affecting sticking
- Establish monitoring and control strategies
Site-Specific Factor Assessment:
When transferring between manufacturing sites:
Evaluate differences:
- Tablet press models and condition
- Environmental control systems
- Water quality (for granulation)
- Raw material sources/suppliers
- Personnel training and experience
Risk mitigation:
- Conduct side-by-side comparisons when possible
- Transfer validated process parameters precisely
- Plan for potential adjustments
- Maintain communication between sites during transfer
Monitoring and Continuous Improvement
Solved sticking problems can recur; systematic monitoring prevents regression and enables optimization.
Key Process Indicators
Metrics to Track Routinely:
Direct Sticking Indicators:
Cleaning Frequency:
- Record punch cleaning intervals
- Trend over time
- Establish baseline for “normal” formulation behavior
- Alert threshold: Cleaning frequency increases >50% from baseline
Punch Inspection Findings:
- Document routine inspection results
- Categorize: No issues / Minor accumulation / Significant sticking / Severe
- Track by formulation, press, tooling set
Tablet Quality Metrics:
- Embossing clarity/completeness
- Surface defects attributed to sticking
- Batch rejection rates
Production Efficiency:
- Actual vs. planned production rate
- Downtime attributed to sticking
- Yield losses from sticking-related defects
Indirect/Leading Indicators:
Environmental Conditions:
- Temperature and humidity trends
- Excursions from setpoints
- Seasonal patterns
Material Properties:
- Granule moisture content trends
- Granule size distribution
- Lubricant concentration verification
Process Parameters:
- Compression force consistency
- Speed variations
- Pre-compression settings
Equipment Condition:
- Tablet press maintenance status
- Tooling wear assessments
- Coating condition evaluations
Data Management:
Implement systematic data collection:
- Electronic batch records capturing key parameters
- Environmental monitoring systems with data logging
- Statistical process control charts for trending
- Correlation analysis between variables
Analysis approach:
- Regular review (weekly/monthly depending on frequency)
- Identify trends before problems manifest
- Correlate sticking incidents with parameter changes
- Use data to optimize process understanding
Validation Approaches
Confirming Solution Effectiveness:
When implementing sticking solutions, validate success systematically:
Validation Protocol:
1. Define Success Criteria:
- Measurable targets: e.g., “Cleaning frequency <1 per 8-hour shift”
- Quality specifications: e.g., “Zero tablets with incomplete embossing”
- Production metrics: e.g., “Maintain target production rate ±5%”
2. Monitoring Period:
- Minimum 3 batches for initial assessment
- Extended monitoring (10+ batches) for confidence
- Document all relevant parameters
3. Comparison to Baseline:
- Pre-implementation metrics vs. post-implementation
- Quantify improvement
- Statistical significance where appropriate
4. Stability of Improvement:
- Verify sustained performance over time
- Assess under various conditions (different seasons, material lots, etc.)
- Ensure robustness
Documentation Requirements:
Create comprehensive records:
Sticking Investigation Report:
- Problem description and history
- Diagnostic approach and findings
- Root cause determination
- Solutions considered
- Selected solution and rationale
- Implementation details
- Validation results
- Lessons learned
Change Control:
- Formal documentation of process/formulation/tooling changes
- Justification and approval
- Impact assessment
- Implementation plan
- Success criteria
- Follow-up monitoring
Continuous Improvement:
- Incorporate learnings into standard procedures
- Update training materials
- Share knowledge across products/sites
- Build institutional knowledge base
Regulatory Considerations:
For marketed products:
- Assess if changes require regulatory notification (variations, supplements)
- Document within quality system (GMP requirements)
- Maintain change history for inspections
- Ensure changes validated appropriately
For development products:
- Document thoroughly for eventual regulatory filings
- Demonstrate control strategy development
- Show design space understanding
Common Questions About Tablet Sticking
What is the most common cause of tablet sticking?
Inadequate or improper lubrication represents the single most frequent cause of tablet sticking. When lubricant concentration is too low, distributed unevenly, or the wrong type is selected for the formulation, friction between the tablet and punch faces increases dramatically, leading to material adhesion. However, tablet sticking is fundamentally multifactorial—moisture content, API properties, compression parameters, tooling condition, and environmental factors all contribute. Effective troubleshooting requires systematic evaluation of all potential causes rather than assuming any single factor is responsible.
Can I fix sticking without reformulating?
Yes, in most cases. The majority of sticking problems can be resolved through combinations of process optimization, environmental control, and tooling modifications without changing the fundamental formulation. Process adjustments (compression speed, force), enhanced environmental control (temperature and humidity), improved tooling maintenance, and anti-stick coatings frequently eliminate sticking. Even minor formulation adjustments within existing specifications (increasing lubricant concentration, extending mixing time) often suffice. Reformulation becomes necessary only when the API or critical excipients have inherent properties that cannot be managed through these approaches, or when all other interventions fail to provide sustainable solutions.
How do I know if I need to recoat my tooling?
Several indicators suggest tooling recoating is necessary. If sticking suddenly worsens despite unchanged formulation and process parameters, coating wear is likely. Microscopic inspection revealing visible coating wear, color changes on punch faces, or exposed substrate indicates coating failure. When previously successful coatings no longer prevent sticking after extensive use (typically 100,000-500,000+ tablets depending on coating type and formulation abrasiveness), recoating should be considered. Additionally, if you’re experiencing persistent sticking with uncoated or standard tooling, proactive coating application often provides the most cost-effective solution compared to ongoing production disruptions.
What’s the difference between chromium nitride and titanium nitride coatings?
Chromium nitride (CrN) and titanium nitride (TiN) serve different primary purposes. CrN excels at anti-stick performance with lower coefficient of friction (0.4-0.5 vs. 0.5-0.7), superior lubricity, and better release characteristics for sticky formulations. CrN also provides excellent corrosion resistance and maintains effectiveness in humid environments. TiN offers superior hardness (2000-2400 HV vs. 1600-2000 HV) and wear resistance, making it ideal for abrasive formulations where tooling wear is the primary concern. For pure sticking prevention, chromium nitride generally performs better. For maximum tooling life with abrasive materials, titanium nitride is preferred. Selection depends on whether anti-stick performance or wear resistance is the greater priority.
How much magnesium stearate is too much?
For most formulations, magnesium stearate concentrations above 1.0% risk negative effects without additional anti-stick benefits. The typical effective range is 0.25-1.0%, with 0.5-0.75% optimal for many applications. Excessive magnesium stearate (>1.0-1.5%) causes reduced tablet hardness and tensile strength, prolonged disintegration and dissolution times, and potential interference with API release—particularly problematic for immediate-release formulations. While increasing lubricant concentration helps sticking, use the minimum effective amount. If 1.0% magnesium stearate doesn’t adequately prevent sticking, consider alternative solutions (different lubricant, tooling coating, process optimization) rather than increasing concentration further.
Should I increase or decrease compression force when experiencing sticking?
The answer depends on the root cause. If tablets are soft and weak, indicating insufficient compression force, increasing force typically helps by creating stronger cohesive bonds within the tablet that resist separation and adhesion to punches. However, if excessive compression force is generating heat (especially with low-melting APIs) or creating high friction, reducing force may alleviate sticking. Start by assessing tablet quality: if tablets are soft with poor hardness, increase force incrementally while monitoring sticking and quality. If tablets are already hard and sticking accompanies heat generation or tablet defects like capping, decrease force. The optimal approach involves creating a compression profile—plotting force against tablet hardness, friability, and sticking severity—to identify the “sweet spot” that provides required quality with minimal sticking.
How does humidity affect tablet sticking?
Relative humidity creates profound sticking effects through moisture adsorption and capillary bridge formation. When atmospheric moisture adsorbs onto particle and tooling surfaces during compression, thin water films form capillary bridges between granules and punch faces. These liquid bridges dramatically increase adhesive forces, causing material to stick rather than releasing cleanly. Hygroscopic formulations are especially vulnerable, rapidly absorbing moisture even during short exposure to humid air. Each formulation has a critical humidity threshold above which sticking worsens dramatically—typically 45-60% RH for standard formulations, lower for hygroscopic materials. Environmental control maintaining humidity below this threshold represents essential sticking prevention for moisture-sensitive products. Even non-hygroscopic formulations show improved release at controlled humidity (40-50% RH) compared to uncontrolled conditions.
Can tablet design contribute to sticking?
Absolutely. Tablet design significantly impacts both sticking and picking propensity. Deep cup profiles increase contact area between formulation and punch faces, create softer tablet cores more prone to adhesion, and limit space for embossing without excessive cup depth. Complex embossing—especially decorative serif fonts, intricate logos, or numerous characters—creates recessed areas where powder readily traps and adheres, resulting in picking. Optimal designs for sticky formulations use shallow cup profiles (standard concave or flat face with bevel), simple sans serif fonts with adequate stroke thickness (>0.3mm), minimal character count, and appropriate letter height (>2-3mm). When brand requirements demand complex design, compensatory measures (pre-picking, advanced coatings, compound cup configurations) become necessary. Engaging tooling specialists early in design prevents manufacturability problems.
When should I use a coating on my punches?
Proactive coating specification is recommended when formulations contain known sticky APIs (low melting point, hygroscopic, thermoplastic properties), require complex embossing increasing picking risk, or have historical evidence suggesting sticking propensity. For high-value products, coating investment provides insurance against production disruptions. Reactive coating becomes appropriate when formulation and process optimization fail to adequately control sticking, production delays justify coating investment, or development timeline allows coating implementation. Even if development tooling performs adequately uncoated, consider coating production tooling due to longer production runs, higher speeds, and commercial scale manufacturing stresses. Cost-benefit analysis typically favors coating for any formulation showing sticking tendency, as production downtime costs quickly exceed coating investment.
How long do anti-stick coatings last?
Coating lifespan varies significantly based on coating type, formulation abrasiveness, and operating conditions. Standard TiN and CrN coatings typically last 50,000-200,000 tablets with moderately abrasive formulations. Advanced coatings (CN+, DLC) extend life to 100,000-500,000+ tablets. Hard chrome shows shorter life (25,000-100,000 tablets) but lower initial cost. Highly abrasive formulations accelerate wear significantly. Signs of coating degradation include return of previously resolved sticking, visible wear patterns or color changes, increased friction during compression, and microscopic evidence of coating delamination. Most coatings can be stripped and reapplied multiple times, making recoating cost-effective for quality punch sets. Establish coating inspection protocols based on tablet count or time intervals to proactively identify degradation before severe sticking recurs.
What are the signs that granules are too moist?
Multiple indicators suggest excessive granule moisture. Tablets feel soft and compress easily, with lower-than-expected hardness values despite adequate compression force. Sticking progressively worsens throughout batch as moisture accumulated on punch faces. Tablets may show surface defects, inconsistent weight (moisture affects flow), or slow dissolution if excess moisture affected granulation properties. The definitive test is loss-on-drying (LOD) or moisture analyzer measurement showing values above specification (typically >2-3% for most formulations). Visual inspection may reveal granules appearing damp, clumping, or flowing poorly. If moisture is suspected, test immediately and compare against established specifications. High moisture requires additional drying before compression or, if discovered during compression, batch disposition decision based on moisture impact.
Can environmental temperature cause sticking?
Yes, particularly for formulations containing low-melting APIs or thermoplastic excipients. Compression generates significant frictional heat; when combined with elevated ambient temperature, formulation temperature may approach or exceed softening points of certain components. APIs like ibuprofen (MP 75-78°C) are especially vulnerable—compression room temperature of 25°C plus frictional heating can create localized temperatures causing thermoplastic flow and adhesion. Even formulations without extremely low-melting components show temperature sensitivity. Standard compression room temperature of 20-25°C works for most formulations, but heat-sensitive materials require 18-22°C or even 16-20°C for very sensitive products. Summer production often shows worse sticking than winter due to ambient temperature effects. Temperature control, compression speed reduction (less frictional heat), and coatings optimized for thermoplastic materials address temperature-related sticking.
Key Takeaways and Action Steps

Systematic Troubleshooting is Essential
Tablet sticking requires diagnostic precision rather than trial-and-error interventions. The most effective approach begins with rapid assessment using the symptom-to-cause mapping framework to identify whether formulation, tooling, process, or environmental factors dominate. This categorization guides efficient solution selection, preventing wasted time on inappropriate interventions.
Most persistent sticking problems involve multiple contributing factors requiring integrated solutions across formulation optimization, tooling enhancement, process control, and environmental management. Documenting all diagnostic findings, interventions, and outcomes builds institutional knowledge that improves future troubleshooting effectiveness and prevents recurrence.
Prioritize Solutions Systematically
Implement solutions in logical order based on speed, cost, and likelihood of success:
Immediate response when production is compromised:
- Reduce compression speed 20-30% to minimize frictional heat
- Verify and optimize environmental conditions (temperature, humidity)
- Inspect tooling for obvious damage requiring immediate cleaning or replacement
- Document all observations and parameter changes
Short-term improvements within days:
- Optimize process parameters (compression force, dwell time)
- Adjust lubrication within specification limits (concentration, mixing time)
- Enhance tooling maintenance (deep cleaning, polishing)
- Implement environmental control enhancements
- Consider gravimetric screening if formulation optimization needed
Long-term sustainable solutions:
- Evaluate and implement anti-stick coatings based on formulation characteristics
- Consider predictive modeling (TSAR) for coating selection
- Assess reformulation if fundamental formulation issues identified
- Develop robust preventive monitoring program
This guide represents current best practices in pharmaceutical tablet sticking troubleshooting based on published research, industry experience, and equipment manufacturer recommendations. Always validate solutions within your specific manufacturing context and regulatory framework.