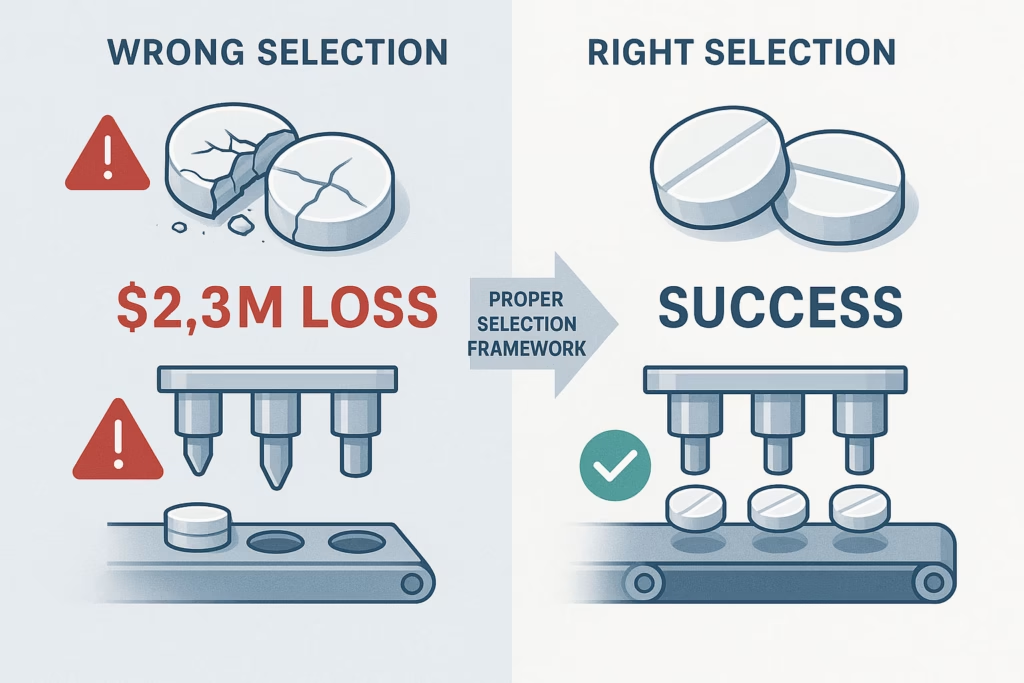
When a major pharmaceutical company recently experienced a $2.3 million production shutdown due to tablet punch sticking issues, it highlighted a critical truth: the wrong tablet punch selection can cost far more than the right one. Despite tablets being the most preferred oral dosage form, with the global market forecast to reach $926.3 billion by 2027, many manufacturers still struggle with systematic approaches to choosing the right tablet punches for their formulation.
The problem isn’t a lack of punch options—it’s the absence of a systematic framework for how to choose the right tablet punches for your formulation. With over 16 different grades of punch steel available and countless size and shape combinations, selecting appropriate tooling can determine the success or failure of a product launch. Understanding how to choose the right tablet punches for your formulation requires a systematic approach that prevents costly production issues and ensures optimal tablet quality.
Understanding Tablet Punch Fundamentals
The Critical Role of Tablet Punches
A tablet is formed by the combined pressing action of two punches and a die, where the upper and lower punches compress granulated material with precise force to create tablets of uniform size and weight. This seemingly simple process involves complex interactions between your formulation, compression forces, and tooling materials.
The Tablet Compression System Components:
• Upper Punch: Applies compression force from above, typically shorter with a guided head design
• Lower Punch: Seals the die from below, controls powder fill depth, and ejects the finished tablet
• Die: Forms the tablet cavity and determines final tablet dimensions
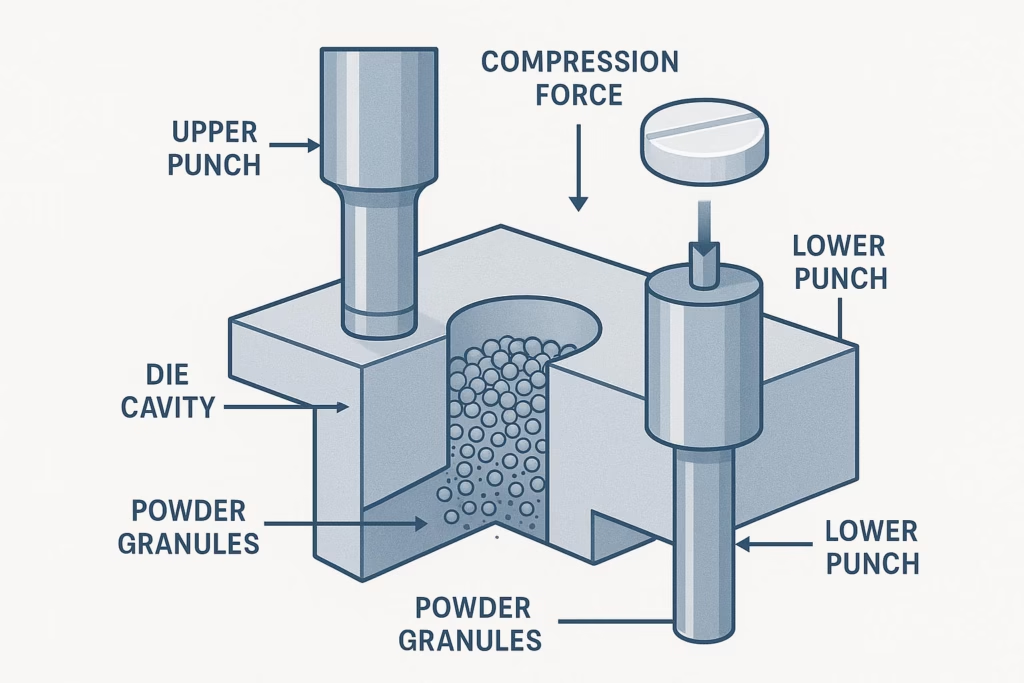
The working length of punches—measured from the head flat to the lowest point of the punch cup—directly determines tablet thickness, weight uniformity, and compression consistency. Even minor variations in punch dimensions can cause significant quality issues.
How Punches Affect Tablet Quality
Your punch selection directly impacts five critical quality attributes. Tablet weight uniformity depends on the lower punch length, which determines how uniformly product fills the die, with deviations affecting both tablet hardness and weight consistency. Mechanical strength is influenced by punch tip geometry, with extra deep concave punches typically producing tablets with lower friability compared to standard configurations. Surface quality requires surface finish below 1μm peak-to-valley height to prevent adhesion, sticking, and filming on tablet surfaces. While punch geometry doesn’t directly affect dissolution rate, it significantly impacts tablet-to-tablet variability in dissolution performance testing. Finally, proper punch selection reduces downtime, minimizes defects, and extends tooling life through appropriate material matching, directly improving production efficiency.
How to Choose the Right Tablet Punches Based on Formulation Analysis
How to Choose the Right Tablet Punches: API Characteristics Assessment
Learning how to choose the right tablet punches for your formulation requires understanding that your Active Pharmaceutical Ingredient (API) properties are the primary driver of punch selection requirements. Conduct this systematic assessment:
Physical Properties Evaluation:
Particle Size and Surface Area API properties such as high surface area, surface energy, and particle size can predispose formulations to manufacturing challenges and significantly impact final dose quality. Fine particles increase adhesion potential, requiring specialized punch materials or coatings.
Moisture Sensitivity Hygroscopic APIs that absorb moisture from the environment create capillary bridges with tooling surfaces, leading to sticking issues. For moisture-sensitive formulations, several strategies are essential: selecting punch materials with lower surface energy, considering hydrophobic coatings, and implementing strict environmental controls during compression.
Thermal Properties APIs with low melting points can cause tablets to melt at punch surfaces under compression pressure. This requires lower compression forces, specialized punch tip treatments, and temperature-controlled manufacturing environments.
Excipient Considerations
Binder Selection Impact Common binders like hydroxypropyl cellulose (HPC), microcrystalline cellulose (MCC), or polyvinyl pyrrolidone (PVP) each interact differently with punch materials, affecting adhesion potential and required compression forces.
Lubricant Optimization
Lubricants reduce friction between tablet surfaces and die walls during ejection, but excessive amounts can cause tablet strength issues and affect disintegration times. The lubricant type and concentration directly influence punch material selection. Magnesium stearate is most common and requires standard steel punches, while stearic acid may require specialized coatings for high-acid formulations. Sodium stearyl fumarate works better for moisture-sensitive formulations.
Choosing the Right Tablet Punches for Abrasive and Corrosive Formulations
Abrasiveness Assessment Aggressive formulations containing hard and sharp elements cause damage to punch tips through repeated compression cycles, leading to surface abrasion and impregnation of particles into the punch tip surface.
Identify abrasive ingredients including crystalline excipients (lactose, mannitol), hard APIs with angular particles, and inorganic salts and oxides.
Corrosive Element Detection Formulations containing chlorine, salts, and acids react with tooling surfaces, resulting in oxidation and premature tool failure. Document any chloride-containing compounds, acidic APIs or excipients, and salt forms that release ions under pressure.
Technical Criteria for Choosing the Right Tablet Punches for Your Formulation
How to Choose the Right Tablet Punches: Tooling Standards Guide
Understanding Standard Classifications
When learning how to choose the right tablet punches for your formulation, understanding tooling standards is crucial. Two main international standards exist: B-tools (EU19/TSM 19) and D-tooling (EU1/TSM 1), classified by barrel diameter, overall length, and punch head diameter. For detailed comparisons, see our comprehensive TSM vs EU standards guide.
B Tooling Specifications:
- Barrel diameter: 0.75 inches (19mm)
- Head diameter: 1 inch (25.4mm)
- Suitable for tablets up to 13mm diameter
- Higher productivity: 20-25% more tablets per turret
D Tooling Specifications:
- Barrel diameter: 1 inch (25.4mm)
- Head diameter: 1.25 inches (31.75mm)
- Suitable for large tablets (up to 25mm diameter)
- Greater compression capability for hard tablets
Selection Decision Matrix:
| Tablet Size | Recommended Tooling | Compression Force | Productivity |
|---|---|---|---|
| < 6mm | B Tooling | Low-Medium | High |
| 6-13mm | B or D Tooling | Medium | Medium-High |
| 13-20mm | D Tooling | Medium-High | Medium |
| > 20mm | D Tooling | High | Lower |
Material Selection for Punches and Dies
Standard Steel Options
Steel selection is a primary factor in determining maximum compression force rating, with heat treatment developing unique characteristics of toughness, wear resistance, and corrosion resistance.
Common Punch Materials:
S7 Tool Steel is ideal for standard applications, offering yield strength of 200,000-250,000 PSI with excellent toughness and wear resistance suitable for most pharmaceutical formulations. D2 Tool Steel contains higher chromium content (12%) providing superior wear resistance, making it recommended for crystalline or hard particle formulations. 440C Stainless Steel features high chromium content (16-18%) with excellent corrosion resistance, required for acidic or chloride-containing formulations. M340 Steel represents premium high-chrome steel with maximum corrosion and wear resistance for the most challenging formulations. For detailed performance comparisons, review our carbide vs steel tablet punches analysis.
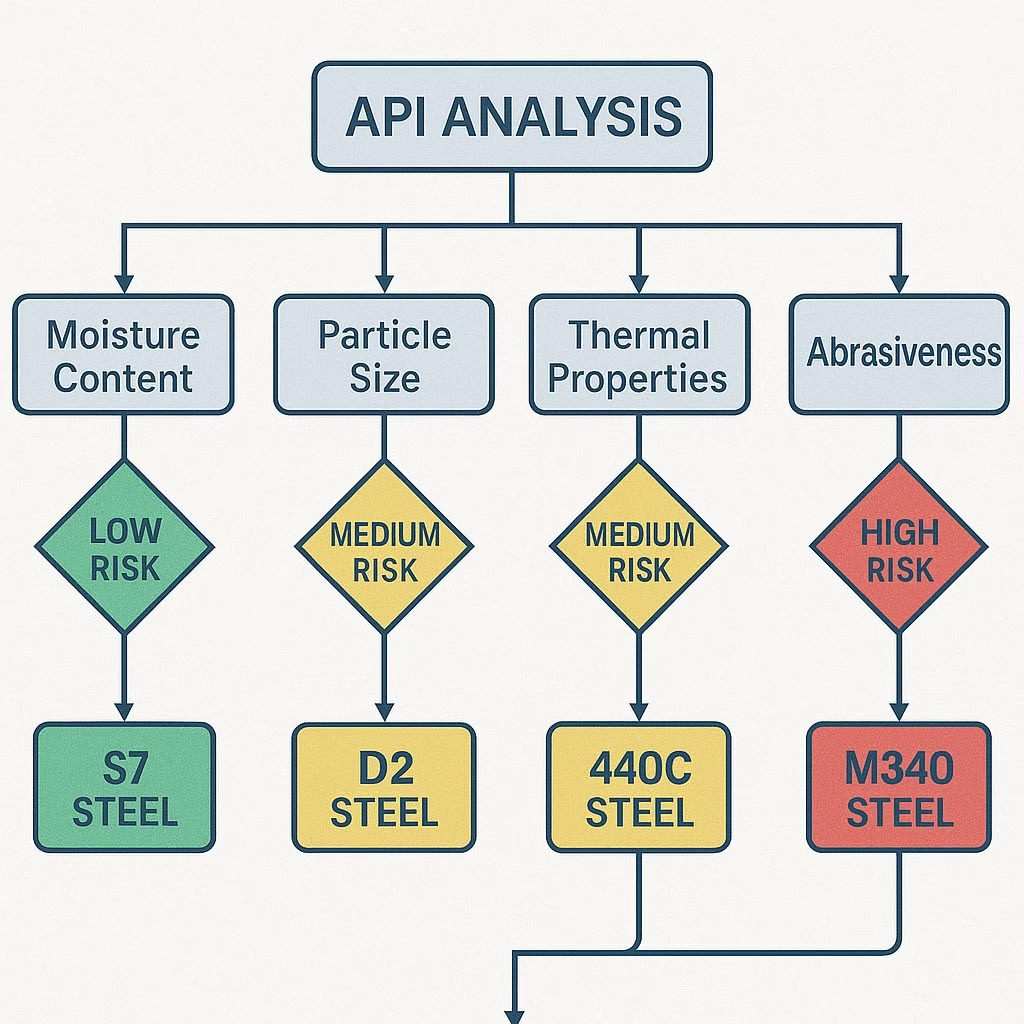
Material Selection Decision Tree:
Formulation Assessment
├── Non-abrasive, non-corrosive → S7 Steel
├── Abrasive ingredients present → D2 or A2 Steel
├── Corrosive elements detected → 440C Stainless
└── Both abrasive AND corrosive → M340 Premium Steel
Size and Shape Considerations
Tablet Weight and Punch Diameter Relationship
The relationship between tablet weight and punch size follows predictable patterns, with typical 350mg tablets requiring specific dimensional considerations.
Standard Size Guidelines show that 100-200mg tablets require 6-8mm diameter punches, 200-400mg tablets need 8-12mm diameter punches, 400-800mg tablets use 12-16mm diameter punches, and tablets over 800mg require 16mm+ diameter punches.
Cup Depth Selection Cup depth directly affects tablet sidewall thickness, with standard tolerances of ±0.003 inch (0.076mm) accepted industry-wide. Shallow cup (C/DIA = 0.07) creates thicker sidewalls but potential coating issues, while standard cup (C/DIA = 0.10) provides balanced performance for most applications. Deep cup (C/DIA = 0.13) reduces sidewall for better tablet aesthetics, and extra deep cup (C/DIA = 0.19) offers lower friability but potential dissolution variability.
Compression Force Requirements
Force Calculation Framework
Rather than comparing compression force alone, calculate compaction pressure by dividing applied force by die area, as punch diameter has an exponential effect on pressure requirements.
Compaction Pressure Formula:
Compaction Pressure (MPa) = Compression Force (N) ÷ Die Area (mm²)
Typical Pressure Requirements vary by formulation type: direct compression requires 50-150 MPa, wet granulation needs 75-200 MPa, dry granulation uses 100-250 MPa, and high-dose APIs require 150-300 MPa.
Maximum Force Limitations The maximum compression force rating depends on steel selection, heat treatment, and tablet configuration, with each tool design being custom-rated for specific applications.
Step-by-Step Framework: How to Choose the Right Tablet Punches
Phase 1: Formulation Property Assessment
Complete this systematic evaluation before considering tooling options:
Formulation Assessment Checklist:
□ API Physical Properties
- Particle size distribution documented
- Moisture content measured
- Thermal properties characterized
- Surface area/energy evaluated
□ Excipient Interaction Analysis
- Binder type and concentration confirmed
- Lubricant selection optimized
- Disintegrant compatibility verified
- Filler abrasiveness assessed
□ Chemical Compatibility Review
- Corrosive elements identified
- pH range established
- Salt formation potential evaluated
- Oxidation susceptibility documented
□ Processing Requirements
- Target tablet weight established
- Hardness specifications defined
- Dissolution requirements documented
- Production volume estimated
Phase 2: Technical Specification Matching
Tooling Standard Selection
Based on your formulation assessment, select appropriate tooling standards:
Decision Logic:
IF tablet_diameter ≤ 13mm AND production_volume = high
THEN select B tooling
ELIF tablet_diameter > 13mm OR compression_force = high
THEN select D tooling
ELIF tablet_weight > 600mg
THEN select D tooling for better compression capability
Material Grade Selection Matrix
| Formulation Risk Level | Recommended Steel Grade | Cost Factor | Tool Life Expectation |
|---|---|---|---|
| Low (standard excipients) | S7 Tool Steel | 1.0x | Standard |
| Medium (some abrasives) | D2/A2 Steel | 1.3x | 150-200% standard |
| High (corrosive elements) | 440C Stainless | 1.8x | 200-300% standard |
| Extreme (abrasive + corrosive) | M340 Premium | 2.5x | 300-400% standard |
Phase 3: Performance Prediction and Validation
Compression Force Calculation
Instead of using compression force alone, normalize for punch tip face area and utilize compaction pressure for scalable formulation development.
Step-by-step calculation:
- Determine target tablet hardness (typically 4-10 kp)
- Calculate required compaction pressure from formulation studies
- Multiply pressure by punch face area for total force requirement
- Verify force is within tooling capability limits
Quality Prediction Methods
Characterizing mechanical properties using compaction emulators with <1 gram samples can predict full-scale manufacturing performance, including:
- Tablet hardness vs. compression force relationships
- Ejection force requirements
- Sticking propensity assessment
- Dissolution performance prediction
Common Problems and Solutions
Sticking and Picking Prevention
Root Cause Analysis
Sticking occurs when adhesive forces between formulation and punch tips overcome cohesive forces within the tablet. Primary causes include:
- Moisture-Related Sticking
- Capillary bridges form between granules and tooling surfaces when moisture content is excessive
- Solution: Environmental control, moisture-resistant excipients
- Insufficient Lubrication
- Inadequate lubricant concentration or improper mixing reduces effectiveness
- Solution: Optimize lubricant level (0.25-1.0%), ensure final-stage blending
- Surface Energy Mismatch
- High-energy formulations adhering to standard steel surfaces
- Solution: Specialized punch coatings or high-chrome steel grades
Preventive Strategies
Environmental Controls: Maintain less than 50% relative humidity in compression areas and control temperature to prevent melting of thermosensitive excipients like macrogol or magnesium stearate. Installing HVAC systems with precise humidity control is essential when choosing the right tablet punches for moisture-sensitive formulations.
Formulation Optimization:
When determining how to choose the right tablet punches for your formulation, select excipients, binders, and fillers with lower moisture content, avoid hygroscopic ingredients when possible, and add anti-adherent agents (colloidal silica, talc) when needed.
Tooling Solutions: Apply specialized coatings (TiN, CrN, DLC), polish punch tips to less than 0.1μm surface roughness, and consider tapered dies for easier tablet ejection. These solutions are critical when learning how to choose the right tablet punches for challenging formulations. For comprehensive information on coating options, see our tablet press coatings comparison guide.
Wear and Maintenance Considerations
Punch Inspection Protocol
Establish a punch inspection program that verifies all punch lengths and cups are within dimensional tolerances to prevent tablet defects.
Critical Measurements:
- Working length tolerance: ±0.002 inch (0.051mm)
- Cup depth tolerance: ±0.003 inch (0.076mm)
- Surface roughness: <1μm peak-to-valley height
- Concentricity: Within 0.001 inch total runout
Maintenance Schedule:
- Daily: Visual inspection for chips, cracks, surface buildup
- Weekly: Dimensional verification of random sample (10% of set)
- Monthly: Complete set measurement and documentation
- Quarterly: Surface roughness evaluation and polishing if needed
Tool Life Extension Strategies
Understanding how to choose the right tablet punches for your formulation includes implementing proper storage in moisture-controlled environments with protective coatings, establishing rotation schedules to systematically rotate punches for even wear, using appropriate cleaning protocols with suitable solvents while avoiding abrasive methods, and professional reconditioning through tip polishing and surface treatment renewal. For detailed maintenance protocols, refer to our tablet punch life extension guide.
Troubleshooting Tablet Defects
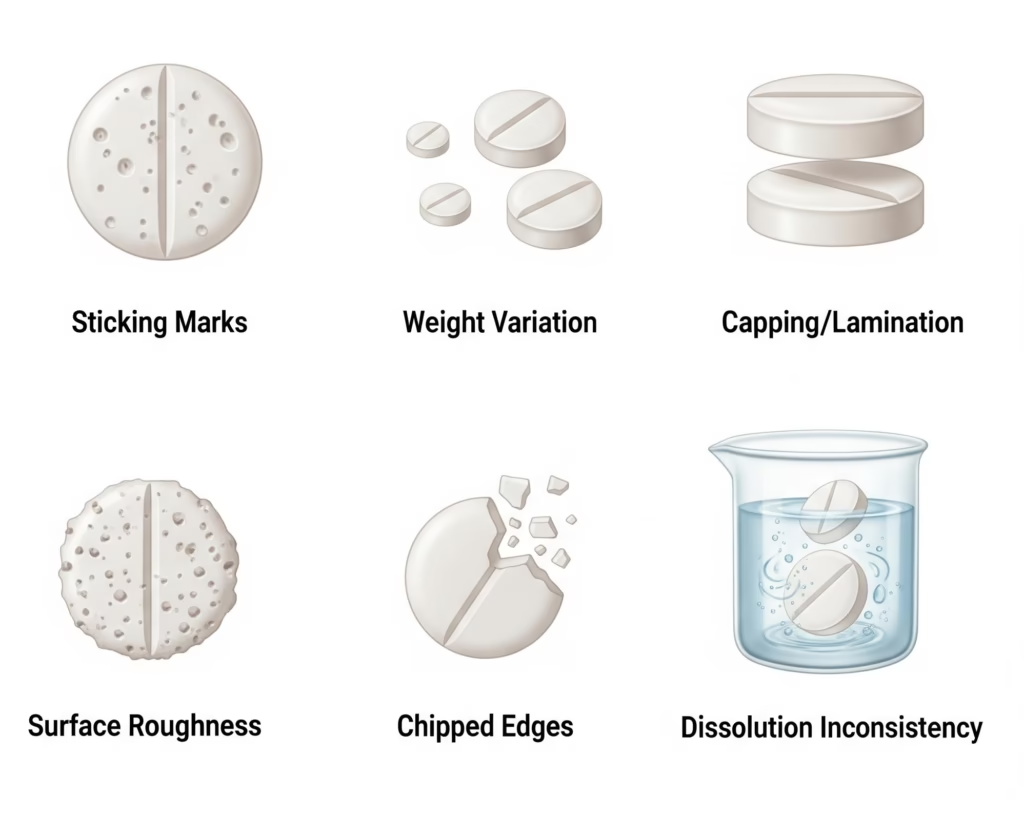
Common Defect-Tooling Relationships
| Tablet Defect | Likely Tooling Issue | Recommended Solution |
|---|---|---|
| Weight variation | Punch length inconsistency | Re-match punch sets, verify working lengths |
| Hardness variation | Worn punch tips, cup depth variation | Measure and replace out-of-spec punches |
| Capping/lamination | Excessive compression force or speed | Reduce force, optimize precompression |
| Sticking/picking | Surface roughness, material incompatibility | Polish tips, upgrade steel grade |
| Chipping/cracking | Punch material too brittle for application | Switch to tougher steel grade |
| Poor dissolution | Inconsistent compression due to tool wear | Replace worn tooling, verify cup depths |
Advanced Considerations for Choosing the Right Tablet Punches
Multi-Tip Punch Applications
When to Consider Multi-Tip Tooling
Multi-tip punches are a cost-effective means of increasing tablet output without adding additional tablet presses, particularly beneficial for:
- High-volume production requirements
- Small tablet sizes (<6mm diameter)
- Formulations with good flow properties
- Applications requiring identical tablet pairs
Design Considerations
Key factors when selecting multi-tip configurations include tool type (B or D), tablet size, number of tips, and particle/powder characteristics:
Tip Configuration Options:
- 2-tip: 50% output increase, good for most applications
- 4-tip: 200% output increase, requires excellent powder flow
- 6-tip: 400% output increase, specialized applications only
Critical Success Factors:
- Uniform die filling across all tips requires optimized feeder systems and fill cam selection
- Increased ejection forces require enhanced lubrication
- More complex cleaning and maintenance procedures
Specialized Coatings and Treatments
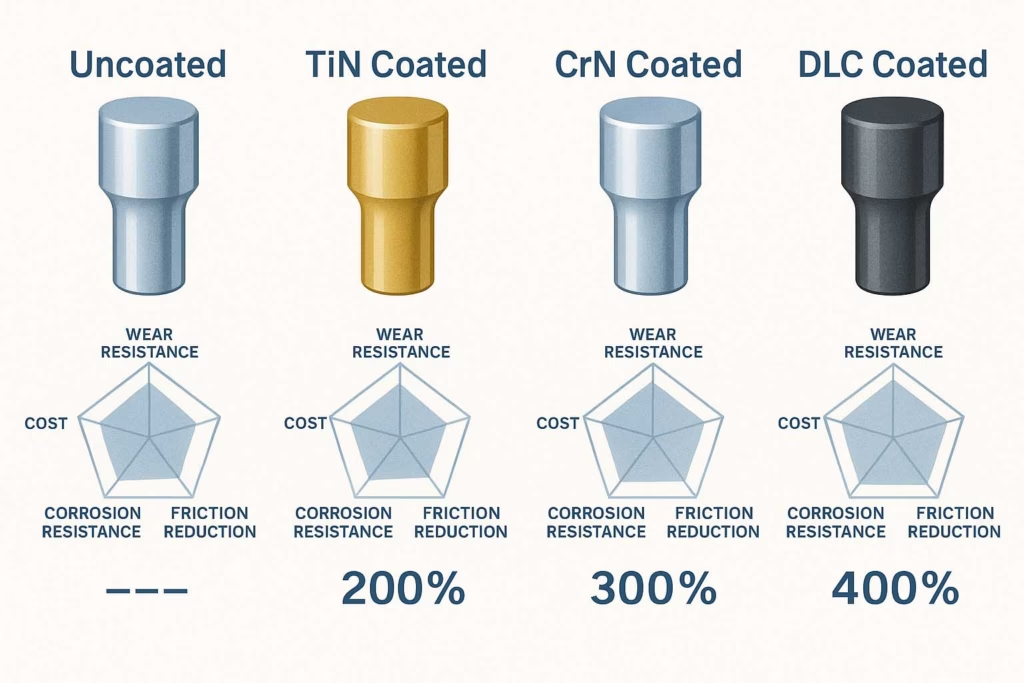
Advanced Surface Technologies
Modern pharmaceutical manufacturing increasingly relies on specialized punch treatments. Titanium Nitride (TiN) Coating reduces friction and adhesion while extending tool life 200-400% with its distinctive gold color for easy identification. Chrome Nitride (CrN) Coating provides superior corrosion resistance while maintaining sharp detail definition with a silver-gray appearance. Diamond-Like Carbon (DLC) Coating offers the lowest friction coefficient with exceptional wear resistance suitable for most challenging formulations. When learning how to choose the right tablet punches for your formulation, specify coatings for chronic sticking issues, highly abrasive formulations requiring extended tool life, corrosive formulations where stainless steel isn’t sufficient, and high-volume production requiring minimal downtime. Learn more about high-performance tablet punch materials and coatings.
Scale-Up Considerations
Laboratory to Manufacturing Translation
Formulation development using small-scale equipment must account for differences in compression speed, dwell time, and force application when scaling to production presses.
Critical Scale-Up Parameters:
- Dwell time differences: Production presses have shorter compression times
- Force application rates: Higher speeds may require formulation adjustments
- Environmental variations: Production environments differ from laboratory conditions
- Batch size effects: Larger batches may show different powder flow characteristics
Validation Protocol:
- Characterize formulation on laboratory press with target tooling
- Conduct pilot-scale trials with identical punch/die specifications
- Verify tablet quality attributes match laboratory results
- Optimize production parameters while maintaining tooling selection
- Establish ongoing quality monitoring for tooling wear effects
Implementation Success Framework
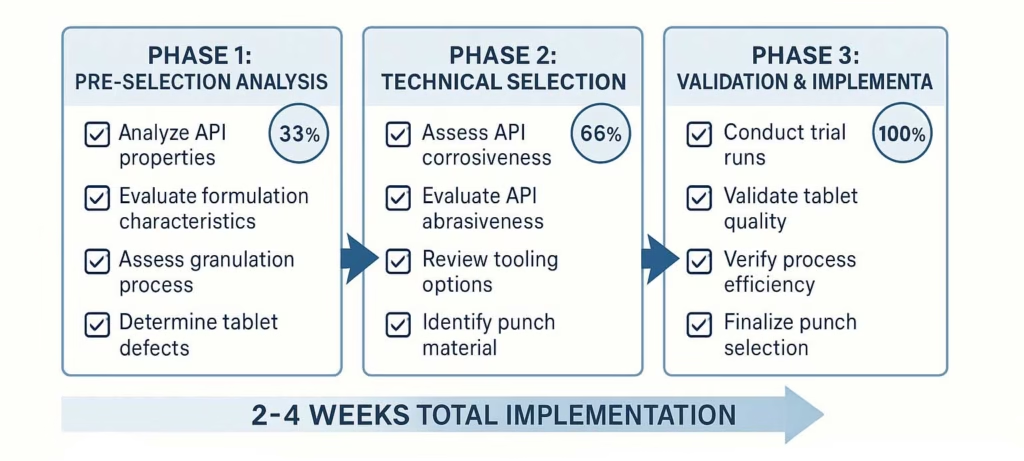
Selection Decision Checklist
Phase 1: Pre-Selection Analysis □ Formulation characterization complete (particle size, moisture, abrasiveness) □ Target tablet specifications defined (weight, hardness, dimensions)
□ Production volume and speed requirements established □ Regulatory requirements and compendial standards reviewed □ Budget parameters and tooling lifecycle costs calculated
Phase 2: Technical Selection □ Tooling standard selected (B vs D) based on tablet size and volume □ Punch material grade chosen based on formulation compatibility □ Cup depth and tip geometry optimized for tablet requirements □ Compression force requirements calculated and verified within limits □ Specialized coatings or treatments specified if needed
Phase 3: Validation and Implementation
□ Laboratory trials conducted with selected tooling configuration □ Tablet quality attributes verified against specifications □ Tool life and maintenance requirements established □ Scale-up protocol developed for production implementation □ Ongoing monitoring and quality control procedures defined
Quality Assurance Integration
Establish these ongoing monitoring protocols:
Daily Monitoring:
- Visual inspection of punch tips for wear or buildup
- Tablet weight and hardness trend analysis
- Production efficiency metrics (downtime, defect rates)
Weekly Analysis:
- Statistical process control of tablet quality attributes
- Punch dimension verification (random sampling)
- Cleaning and maintenance effectiveness review
Monthly Evaluation:
- Complete tooling set dimensional analysis
- Tool life prediction and replacement planning
- Cost-benefit analysis of current selection vs. alternatives
Conclusion
Selecting the right tablet punches for your formulation is a systematic process that directly impacts product quality, production efficiency, and manufacturing costs. The framework presented here provides a comprehensive approach that moves beyond trial-and-error methods to evidence-based tooling decisions for pharmaceutical manufacturers seeking to optimize their formulation processes.
Key Success Factors for Choosing the Right Tablet Punches:
Thorough Formulation Analysis represents the foundation when learning how to choose the right tablet punches for your formulation, as understanding your API and excipient properties drives all subsequent decisions. Technical Specification Matching through systematic evaluation of tooling standards, materials, and dimensions ensures optimal performance for your specific formulation requirements. Problem Prevention by addressing potential issues during the selection process prevents costly production disruptions and quality failures. Ongoing Optimization through regular monitoring and maintenance maximizes tool life and tablet quality while adapting to evolving formulation needs.
Understanding how to choose the right tablet punches for your formulation represents a critical manufacturing competency. The pharmaceutical industry’s continued growth demands manufacturing excellence, and proper tablet punch selection forms the foundation of achieving that goal. By implementing this systematic approach, pharmaceutical manufacturers can ensure their formulation development and manufacturing processes achieve technical excellence and regulatory compliance.
Remember that learning how to choose the right tablet punches for your formulation is not a one-time decision but an ongoing optimization process. As formulations evolve and production requirements change, revisiting these selection criteria ensures continued manufacturing success and product quality excellence.
For expert consultation on complex formulations or challenging applications, professional tooling consultation can prevent costly mistakes and optimize your tablet punch selection process. This comprehensive guide represents current industry best practices and should be validated through appropriate testing protocols for your specific formulation requirements. Additional resources include the FDA’s Pharmaceutical cGMP Guidance and ICH Q8 Quality by Design guidelines for systematic approach validation.