- What Exactly Are Tablet Punches and Dies?
- Understanding the Different Types of Tablet Compression Tooling
- The Science Behind Materials and Manufacturing
- Navigating International Standards: TSM, EU, and ISO
- Mastering Selection Criteria for Optimal Performance
- Solving Common Problems and Troubleshooting Issues
- Implementing Effective Maintenance and Lifecycle Management
- Optimizing Costs and Maximizing ROI
- Ensuring Regulatory Compliance and Quality Excellence
- Embracing Future Trends and Industry 4.0 Technologies
- Mastering Supplier Selection and Procurement
- Your Path to Tablet Tooling Excellence
- Your Journey to Tooling Mastery Begins Now
- Related Articles
Picture this: You’re standing in your pharmaceutical manufacturing facility, watching thousands of tablets roll off the production line every hour. Each tablet looks identical, weighs exactly what it should, and meets every quality specification. Behind this seamless operation lies a technology that most people never think about—tablet punches and dies.
These precision-engineered tools are the unsung heroes of pharmaceutical manufacturing. They transform powder into the medications that millions of people depend on daily. Yet despite their critical importance, many manufacturers struggle with tooling-related problems that cost them thousands of dollars in downtime, rejected batches, and regulatory headaches.
Whether you’re a pharmaceutical manufacturer looking to optimize your production, a machine operator troubleshooting quality issues, or a procurement manager evaluating suppliers, this comprehensive guide will give you everything you need to master tablet compression tooling.
What Exactly Are Tablet Punches and Dies?
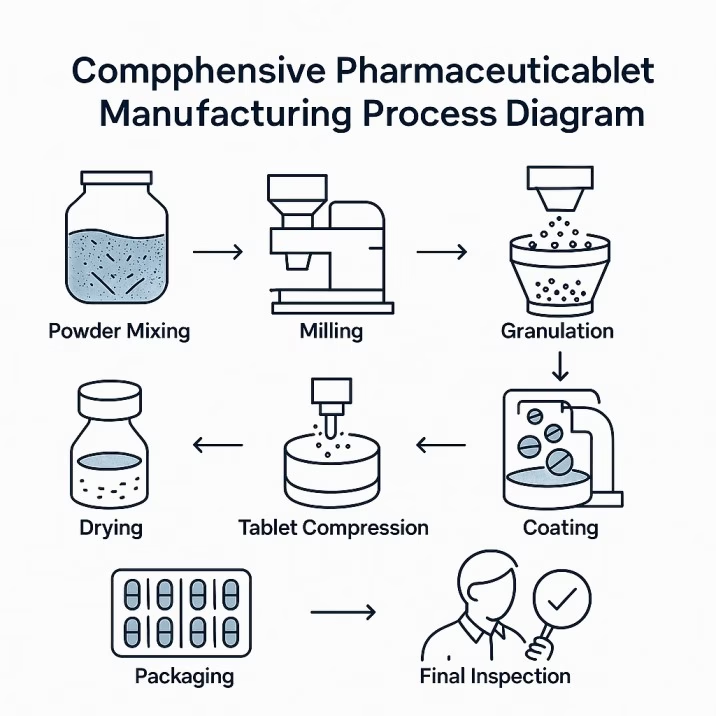
Before diving into the technical complexities, let’s establish the fundamentals. Tablet punches and dies are precision-machined tools that work together in tablet compression machines to transform pharmaceutical powders into solid dosage forms.
Think of this process like a sophisticated sandwich maker. The die is like the bread mold—it creates the outer shape and contains the filling. The upper and lower punches are like the top and bottom plates that compress everything together. But unlike making a sandwich, tablet compression happens with incredible precision, tremendous force, and split-second timing.
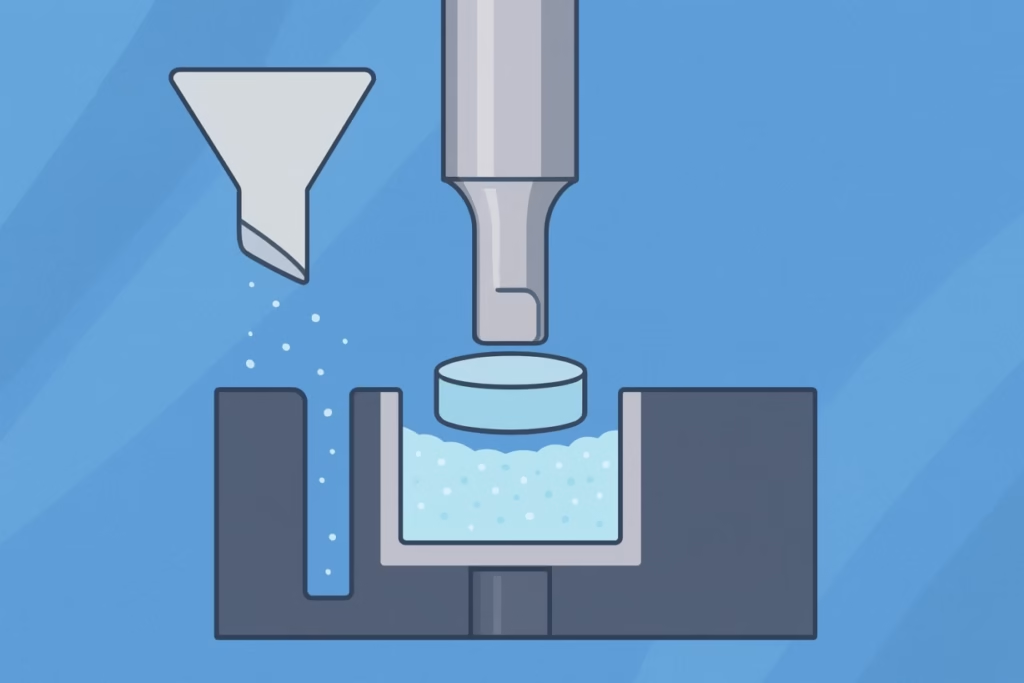
The magic happens in three distinct phases. During the filling phase, carefully measured powder flows into the die cavity while the lower punch sits at just the right depth to create the perfect tablet thickness. Next comes the compression phase, where the upper punch descends with precisely controlled force—sometimes exceeding 50,000 pounds—while the lower punch provides the anvil for compression. Finally, in the ejection phase, the lower punch rises to push the finished tablet out of the die while the upper punch retracts.
This entire process repeats hundreds or even thousands of times per minute on modern tablet presses. The consistency and precision required are staggering when you consider that pharmaceutical tablets must meet weight specifications within 5% tolerance, maintain uniform hardness, and deliver consistent drug release profiles.
The quality of your tablet punches and dies directly impacts every aspect of your tablet’s performance. Poor tooling leads to weight variation, surface defects, sticking problems, and even catastrophic issues like capping and lamination. Conversely, high-quality tooling properly matched to your formulation can increase productivity by 25% while virtually eliminating quality problems.
Understanding the Different Types of Tablet Compression Tooling
Not all tablet tooling is created equal. The pharmaceutical industry has developed several distinct tooling systems, each designed for specific applications and production requirements.
Standard Tooling Classifications
The most fundamental distinction in tablet tooling revolves around the punch diameter and intended application. B-type tooling features a 0.5-inch punch diameter and serves as the workhorse for small to medium tablet production. If you’re manufacturing tablets up to 13mm in diameter or running pilot-scale production, B-type tooling offers the perfect balance of performance and cost-effectiveness.
D-type tooling steps up the game with a full 1-inch punch diameter. This larger, more robust design handles the demands of high-volume production and larger tablets up to 25mm in diameter. The increased surface area and structural strength mean D-type tooling can withstand compression forces up to 100 kN while maintaining dimensional accuracy over millions of compression cycles.
For specialized applications, BB-type and DB-type tooling extend these concepts further. BB-type tooling takes the B-type design and adds extra length for deeper dies, perfect for large tablets that require substantial powder capacity. DB-type tooling combines D-type strength with enhanced length, creating the ultimate solution for maximum powder capacity applications like large nutritional supplements or veterinary tablets.
Multi-Tip Tooling Revolution
One of the most significant productivity innovations in recent years has been the development of multi-tip tooling. Instead of producing one tablet per compression cycle, multi-tip punches can create two, three, or even more tablets simultaneously.

Dual-tip tooling doubles your productivity overnight by producing two identical tablets with each compression stroke. The engineering challenges are significant—powder flow must be perfectly controlled to ensure equal filling of both tablet cavities, and the compression forces must be precisely balanced. However, when properly implemented, dual-tip tooling can transform the economics of tablet manufacturing.
Triple-tip tooling pushes this concept even further, creating three tablets per cycle for a 200% productivity increase. The complexity increases exponentially, requiring extremely precise powder handling and sophisticated process control, but the payoff can be enormous for high-volume products.
Shaped and Custom Tooling
While round tablets dominate pharmaceutical manufacturing, shaped tooling opens up possibilities for improved patient compliance and brand differentiation. Oval tablets provide better swallowing characteristics for many patients, while capsule-shaped tablets can mimic the familiar appearance of capsules while providing the manufacturing advantages of compressed tablets.

Custom embossing adds another dimension to tablet design. Beyond simple identification codes, modern tooling can create complex logos, break lines for accurate dose splitting, and even anti-counterfeiting features. However, custom tooling requires careful consideration of powder flow characteristics and ejection forces to avoid manufacturing problems.
Specialized Applications
Some manufacturing requirements demand truly specialized tooling solutions. Core rod tooling enables the production of tablet-in-tablet products, where a smaller tablet is embedded within a larger one to create sophisticated release profiles. Layered tablet tooling allows for the sequential compression of different formulations to create bi-layer or tri-layer tablets with distinct drug release characteristics.
The Science Behind Materials and Manufacturing
The materials used in tablet punches and dies represent one of the most critical decisions in tooling selection. The wrong material choice can lead to premature wear, product contamination, or catastrophic failure during production.
Steel Grades and Their Applications
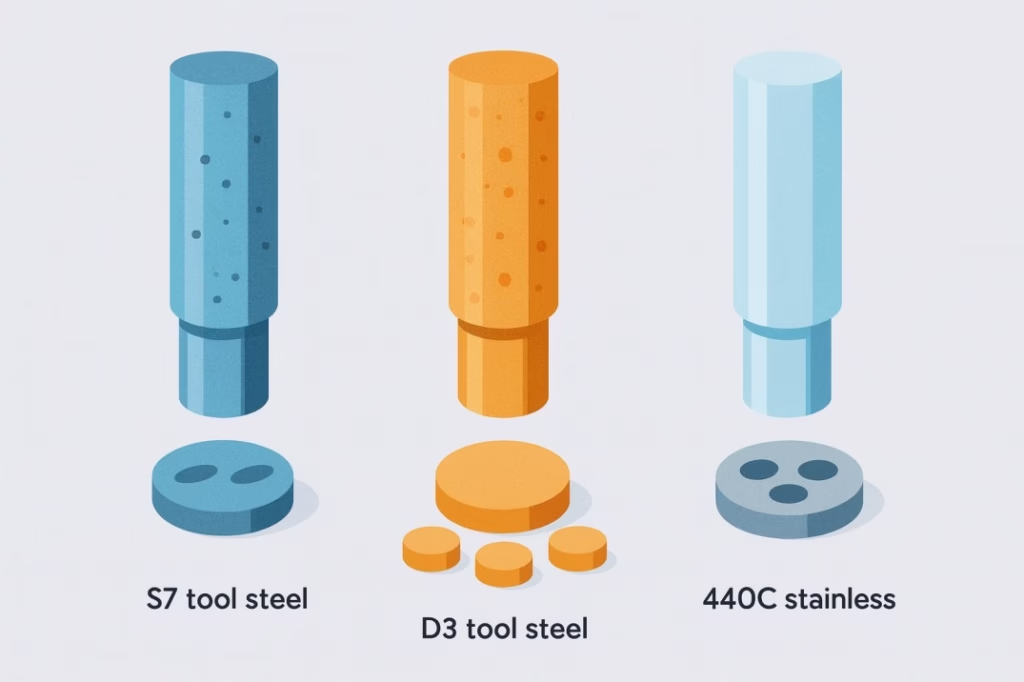
The foundation of most tablet tooling begins with carefully selected tool steels. S7 tool steel serves as the industry standard for general pharmaceutical applications. With its balanced composition of carbon, chromium, and molybdenum, S7 provides excellent toughness and adequate wear resistance for most formulations. The steel achieves a hardness of 58-62 HRC after proper heat treatment, offering the durability needed for hundreds of thousands of compression cycles.
However, not all formulations are created equal. Abrasive ingredients like microcrystalline cellulose, calcium carbonate, or dicalcium phosphate can quickly wear down standard tool steels. For these challenging applications, D3 tool steel offers superior wear resistance thanks to its higher chromium content. The increased hardness and wear resistance can extend tool life by 3-5 times compared to S7 steel, making it cost-effective despite the higher initial investment.
D2 tool steel represents the premium option for demanding applications. Its balanced composition provides excellent wear resistance while maintaining the toughness needed for high-impact compression forces. Many manufacturers find that D2 tooling pays for itself through extended service life and reduced replacement frequency.
For ultimate corrosion resistance, 440C stainless steel becomes the material of choice. While slightly softer than the tool steels, 440C offers exceptional resistance to moisture and corrosive formulation ingredients. This makes it ideal for hygroscopic materials or formulations that require frequent washing and sanitization.
Advanced Materials for Extreme Applications
When standard tool steels reach their limits, advanced materials provide solutions for the most demanding applications. Tungsten carbide represents the ultimate in wear resistance, offering hardness levels that exceed conventional steels by orders of magnitude. A tungsten carbide punch can last 10-15 times longer than standard tool steel in highly abrasive applications.
The economics of tungsten carbide tooling often surprise manufacturers. While the initial cost may be 5-8 times higher than standard steel, the extended service life typically provides a positive return on investment within the first year of use. For high-volume production of abrasive formulations, tungsten carbide tooling becomes not just an option but a necessity for economic manufacturing.
Surface Coating Technologies
Even the best base materials can benefit from advanced surface coatings that provide specialized properties. Physical Vapor Deposition (PVD) coatings represent the current state-of-the-art in tooling surface treatment.
Titanium Nitride (TiN) coating creates a distinctive gold-bronze appearance while providing exceptional hardness and lubricity. The coating thickness of just 2-4 microns doesn’t affect tooling dimensions but dramatically improves performance. TiN-coated punches typically last 2-3 times longer than uncoated tooling while significantly reducing sticking and picking problems.
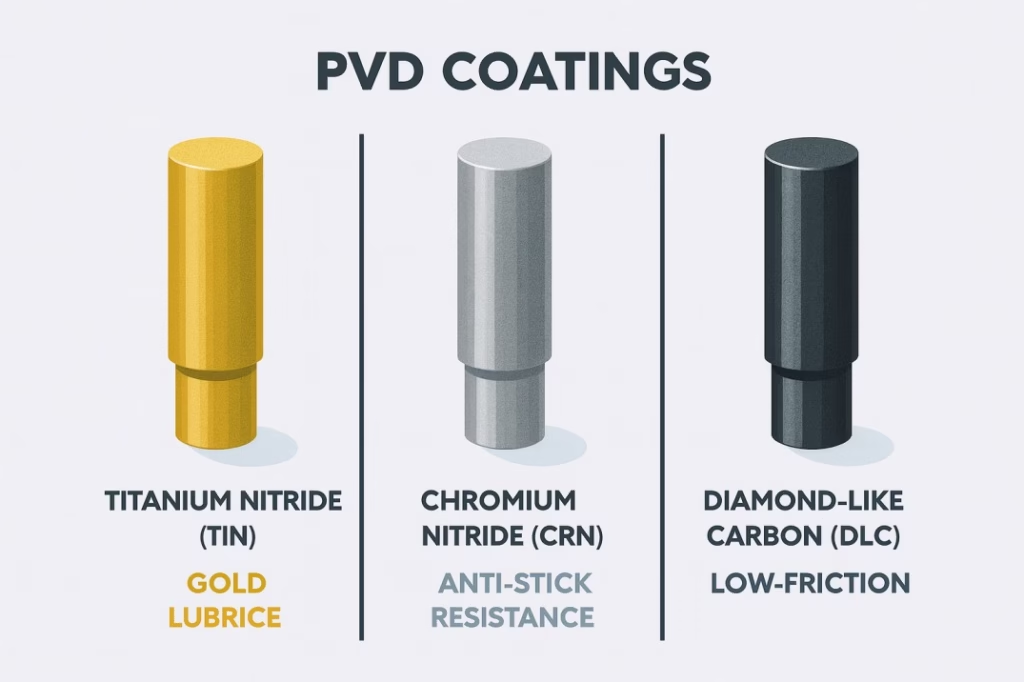
Chromium Nitride (CrN) coating offers superior anti-stick properties, making it the preferred choice for problematic formulations. The silver-gray coating provides excellent hardness while offering better corrosion resistance than TiN. Many manufacturers find that CrN coating virtually eliminates tablet sticking problems that plague uncoated tooling.
Diamond-Like Carbon (DLC) coating represents the ultimate in low-friction performance. With a friction coefficient approaching that of diamond, DLC-coated punches glide through even the most challenging formulations with minimal wear. While more expensive than other coatings, DLC provides the longest service life and best performance for critical applications.
Manufacturing Precision and Tolerances
The precision required in tablet tooling manufacturing would impress even aerospace engineers. Punch diameters must be held to tolerances of ±0.0025mm (±0.0001 inches) to ensure proper fit in tablet press tooling stations. Surface finish requirements typically call for Ra values below 0.1 microns—smooth enough that you couldn’t feel any texture even with the most sensitive instruments.
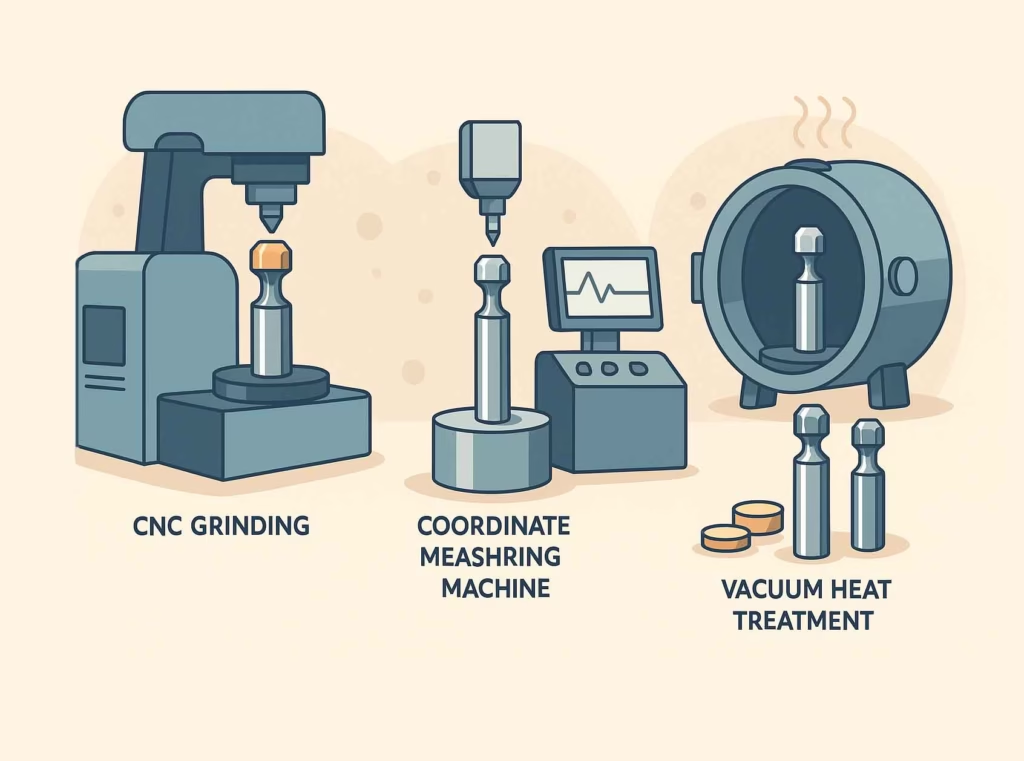
This level of precision requires sophisticated manufacturing equipment and rigorous quality control. Computer-controlled grinding machines work to tolerances measured in fractions of microns, while coordinate measuring machines verify every critical dimension. The heat treatment process must be precisely controlled to achieve the required hardness uniformity, and surface coatings are applied in vacuum chambers with computer-controlled parameters.
Manufacturing standards for tablet tooling often reference ASTM International specifications for material properties and testing procedures. These standardized testing methods ensure consistent quality across different suppliers and manufacturing locations.
Navigating International Standards: TSM, EU, and ISO
One of the most confusing aspects of tablet tooling involves the different international standards that govern tooling dimensions and specifications. Understanding these standards is crucial for anyone involved in global pharmaceutical manufacturing.
The TSM Standard Dominance
The Tablet Specification Manual (TSM), currently in its 7th edition, dominates North American and much of the Asian pharmaceutical market. Developed by the American Pharmacists Association, TSM standards specify everything from punch head angles to die dimensions with incredible precision.
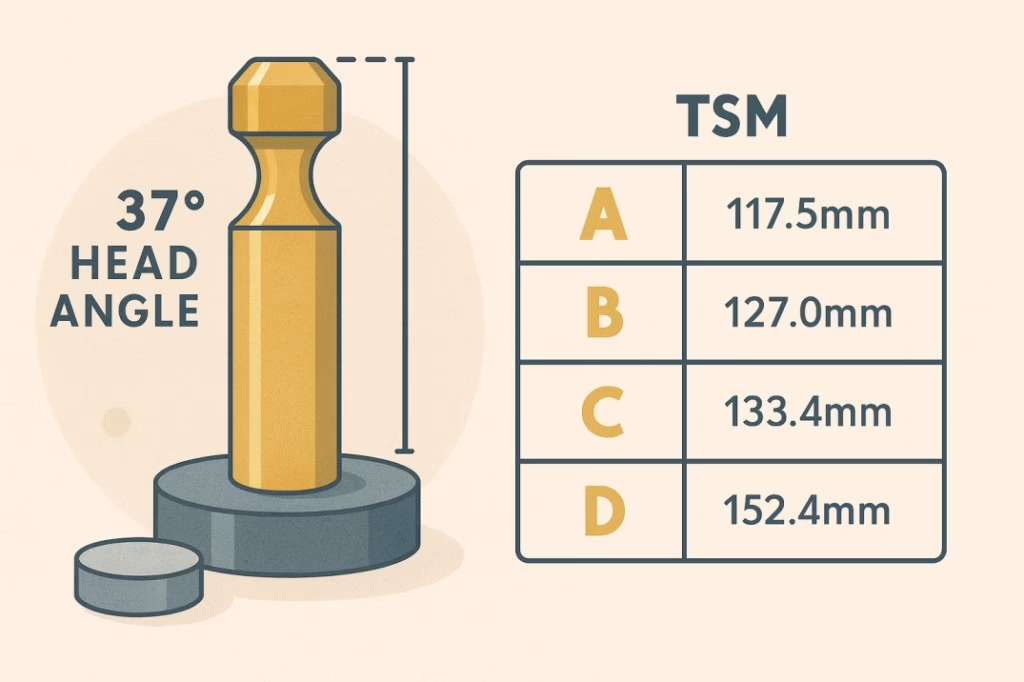
The distinctive 37-degree head angle immediately identifies TSM tooling. This seemingly arbitrary angle actually represents decades of optimization for tablet press mechanics and ejection forces. TSM punch lengths follow a logical progression—A-length punches at 117.5mm for standard applications, B-length at 127.0mm for deeper dies, C-length at 133.4mm for specialized needs, and D-length at 152.4mm for maximum powder capacity.
Die specifications under TSM standards are equally precise. Standard outside diameters range from 6.5mm to 25.4mm in carefully selected increments, while die length typically measures 15.9mm unless custom applications require different dimensions. The bore tolerance specifications of +0.000/-0.005mm ensure consistent tablet dimensions while allowing for thermal expansion during operation.
European Standards and Their Differences
European standards, governed by the European Pharmacopoeia Commission, take a different approach to tablet tooling specifications. The most obvious difference lies in the 30-degree punch head angle, compared to TSM’s 37-degree specification. This seven-degree difference might seem minor, but it’s enough to prevent interchangeability between the two systems.
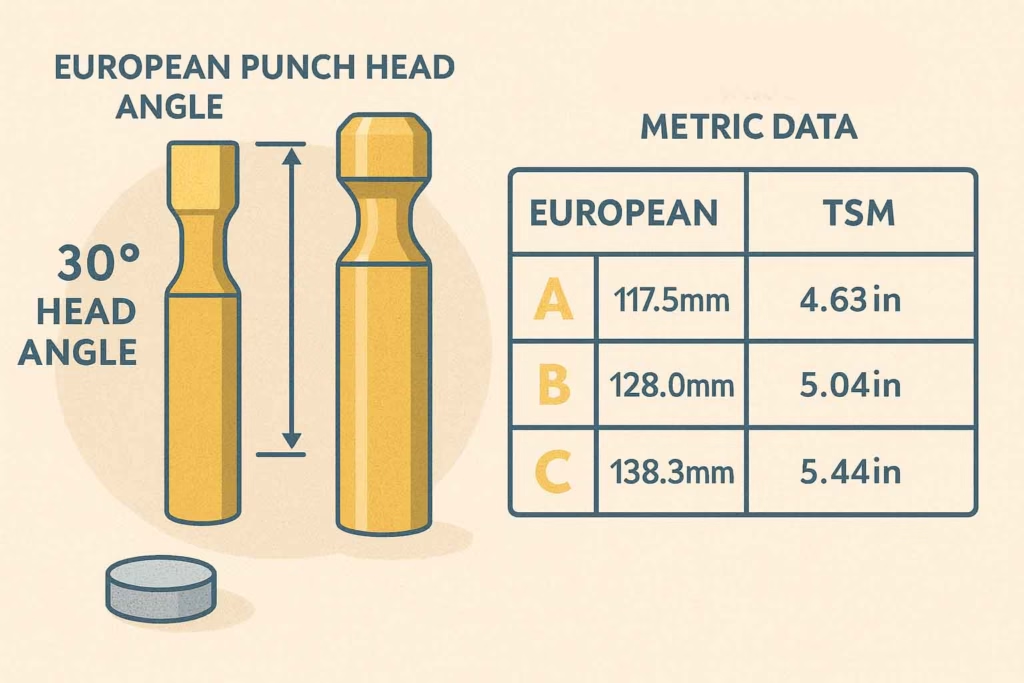
European standards embrace the metric system throughout, with all dimensions specified in millimeters rather than the inch-based TSM system. Length standards, cup dimensions, and tolerances all follow metric conventions, creating a parallel universe of tooling specifications that serves European pharmaceutical manufacturing.
The practical implications of these differences extend beyond simple measurements. European punches won’t fit properly in TSM-specification tablet presses, and vice versa. The different head angles affect ejection forces and can lead to operational problems if the wrong standard is used. Key and keyway dimensions also differ, providing a mechanical safeguard against accidentally mixing standards.
The Coming ISO Harmonization
The International Organization for Standardization (ISO) has been working on ISO 18084, an ambitious attempt to harmonize global tablet tooling standards. Expected for publication in 2025-2026, this standard could revolutionize international pharmaceutical manufacturing by creating truly global tooling interchangeability.

The proposed ISO standard would establish common dimensions, tolerances, and testing procedures that could work across all major tablet press manufacturers. This harmonization could dramatically reduce inventory complexity for multinational pharmaceutical companies while simplifying the supplier base and reducing costs.
However, the transition to ISO standards presents significant challenges. Existing tablet presses are designed around current standards, and retrofitting millions of dollars worth of equipment isn’t economically feasible for most manufacturers. The ISO standard will likely coexist with TSM and European standards for many years, adding another layer of complexity rather than simplifying the situation immediately.
Making Standard Selection Decisions
For new facilities or equipment purchases, standard selection should align with your primary market and equipment suppliers. North American and Asian operations typically benefit from TSM standards due to the larger supplier base and lower costs. European operations naturally gravitate toward European standards due to local equipment and supplier ecosystems.
The economic implications of standard selection extend beyond initial tooling costs. TSM’s larger market share typically translates to more supplier competition and lower prices. Emergency replacement availability also favors TSM in most global markets. However, European standards may offer advantages in specific applications or when working with European equipment manufacturers.
Mastering Selection Criteria for Optimal Performance
Selecting the right tablet punches and dies requires a systematic approach that considers formulation characteristics, production requirements, and economic factors. The wrong choice can lead to quality problems, increased costs, and regulatory compliance issues.
Formulation-Driven Selection
Your formulation characteristics should drive every aspect of tooling selection. Powder abrasivity represents perhaps the most critical factor. Formulations containing microcrystalline cellulose, calcium carbonate, or other abrasive excipients will quickly destroy standard tool steel punches. For these applications, premium materials like D3 tool steel or tungsten carbide become essential for economical operation.
Moisture sensitivity creates another critical consideration. Hygroscopic materials or formulations prone to sticking require specialized surface treatments or materials. Stainless steel construction may be necessary for formulations that require frequent washing or exposure to moisture. Alternatively, specialized coatings like CrN can provide anti-stick properties while maintaining the cost advantages of tool steel construction.
The compression characteristics of your formulation also influence tooling selection. Elastic materials that tend to spring back after compression may require modified punch cup geometries or specialized ejection considerations. Brittle materials prone to capping or lamination might benefit from specific punch face designs or compression profiles.
Sticking and picking tendencies vary dramatically between formulations. Some materials seem to stick to everything, while others compress cleanly with minimal tooling requirements. Understanding your formulation’s behavior allows you to select appropriate surface treatments, coatings, or materials that prevent problems before they occur.
Production Volume Economics
Production volume dramatically affects the economics of tooling selection. Low-volume or research and development operations often prioritize initial cost over long-term durability. B-type tooling with standard materials provides adequate performance at the lowest initial investment for these applications.
Medium-volume production operations typically benefit from D-type tooling with premium materials. The increased initial investment pays dividends through improved durability and reduced replacement frequency. Coating applications become economically attractive when production volumes justify the additional cost.
High-volume operations demand maximum performance regardless of initial cost. Tungsten carbide tooling with premium coatings may cost 10 times more than standard alternatives, but the extended service life and improved productivity often provide positive returns within months. For operations producing millions of tablets annually, tooling costs become insignificant compared to the value of consistent production and quality.
Quality and Regulatory Considerations

Pharmaceutical manufacturing operates under strict quality requirements that directly impact tooling selection. Weight uniformity specifications require consistent tooling dimensions maintained throughout the tool’s service life. Surface finish requirements may dictate specific materials or coatings to achieve the necessary tablet appearance.

Regulatory compliance adds another layer of complexity. FDA validation requirements may specify particular materials or suppliers. Change control procedures can make tooling modifications expensive and time-consuming. International operations must consider multiple regulatory jurisdictions with potentially conflicting requirements.
Cleaning and sanitization requirements vary significantly between applications. Some formulations require frequent tooling cleaning that can damage certain coatings or materials. Others may require sterilization processes that limit material options. Understanding these requirements early in the selection process prevents costly mistakes later.
Economic Optimization Strategies
The total cost of ownership approach provides the most accurate framework for tooling selection decisions. Initial purchase price represents only a small fraction of true tooling costs over the product lifecycle. Tool replacement frequency, maintenance requirements, and productivity impacts often dwarf the initial investment.
Extended tool life through premium materials or coatings typically provides the best return on investment for established products. However, new product development or uncertain production volumes may justify lower initial investments with higher long-term costs. Risk assessment becomes crucial when balancing upfront costs against future uncertainties.
Supplier relationships also impact economic decisions. Long-term partnerships with reliable suppliers may justify premium pricing for superior service and support. Conversely, competitive bidding for standard tooling can reduce costs when performance requirements are well-understood and supplier capabilities are adequate.
Solving Common Problems and Troubleshooting Issues
Even with perfect tooling selection, pharmaceutical manufacturers inevitably encounter problems that require systematic troubleshooting and resolution. Understanding common issues and their solutions can save thousands of dollars in downtime and rejected product.
The Sticking and Picking Challenge
Tablet sticking and picking represents the most common and frustrating problem in tablet manufacturing. When powder adheres to punch faces, it creates surface defects, dimensional variations, and in severe cases, complete tablet destruction. The problem seems simple—material sticks to metal—but the underlying causes and solutions are surprisingly complex.
Moisture plays a central role in most sticking problems. Even small increases in ambient humidity can transform a well-behaved formulation into a sticking nightmare. Hygroscopic ingredients absorb moisture from the air, creating tacky surfaces that readily adhere to tooling. The solution often involves environmental control rather than tooling changes.

Formulation factors contribute significantly to sticking tendencies. Insufficient anti-adherent levels, incompatible excipients, or high-fat content formulations all increase sticking propensity. Sometimes a simple increase in magnesium stearate concentration by 0.1-0.2% eliminates sticking problems entirely.
Tooling surface conditions dramatically affect sticking behavior. Rough surfaces with Ra values above 0.2 microns provide nucleation sites for material adhesion. Worn or damaged punch faces create irregularities that trap powder particles. Even microscopic scratches can initiate sticking problems that progressively worsen over time.
The systematic approach to solving sticking problems begins with immediate actions to restore production. Reducing compression force by 10-20% often provides temporary relief while investigating root causes. Increasing machine speed reduces dwell time and can minimize sticking. Cleaning punches with appropriate solvents removes accumulated deposits that contribute to the problem.
Short-term solutions focus on tooling improvements. Polishing punch faces to Ra values below 0.1 microns often eliminates sticking entirely. CrN coating application provides excellent anti-stick properties for problematic formulations. Sometimes simply replacing worn tooling with new, properly finished punches solves the problem permanently.
Long-term solutions address fundamental causes. Environmental control systems maintain humidity below 50% to prevent moisture absorption. Formulation optimization with improved anti-adherent systems provides robust performance across environmental variations. Premium tooling materials like tungsten carbide with DLC coatings offer ultimate sticking resistance for the most challenging applications.
Capping and Lamination Solutions

Capping occurs when the top or bottom portion of a tablet separates, while lamination involves horizontal splitting through the tablet center. Both problems indicate issues with the compression process or tooling geometry that prevent proper tablet formation.
Capping typically results from excessive elastic recovery during tablet ejection. Deep punch cups concentrate stress at the tablet crown, leading to separation as the tablet expands after compression. Air entrapment during compression can also create weak zones that fail during ejection. Sometimes the problem lies in inadequate bonding between particles due to insufficient compression force or inappropriate formulation characteristics.
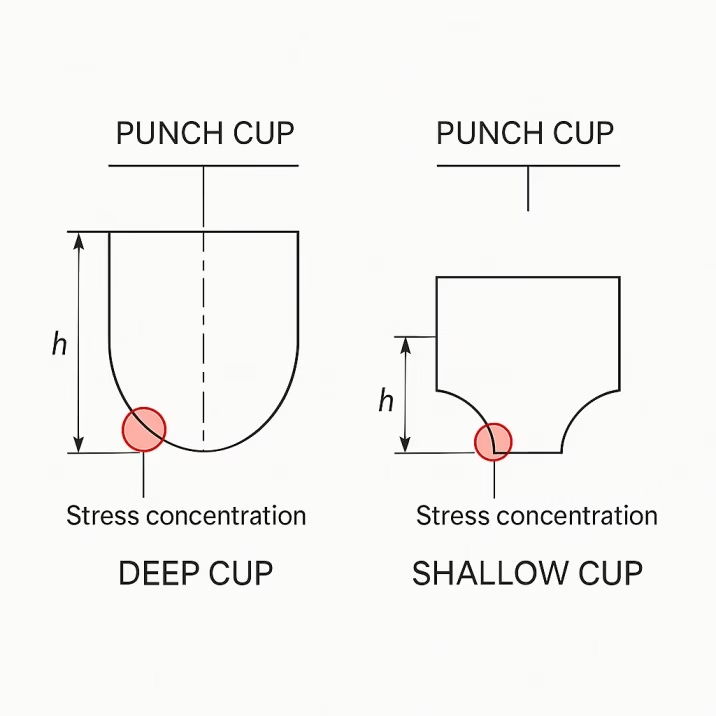
The diagnostic approach begins with careful observation of when failures occur. Capping during ejection suggests tooling geometry issues or excessive ejection forces. Failures during handling indicate fundamental tablet strength problems. Pattern analysis often reveals whether the problem affects all tablets or specific positions on rotary presses.
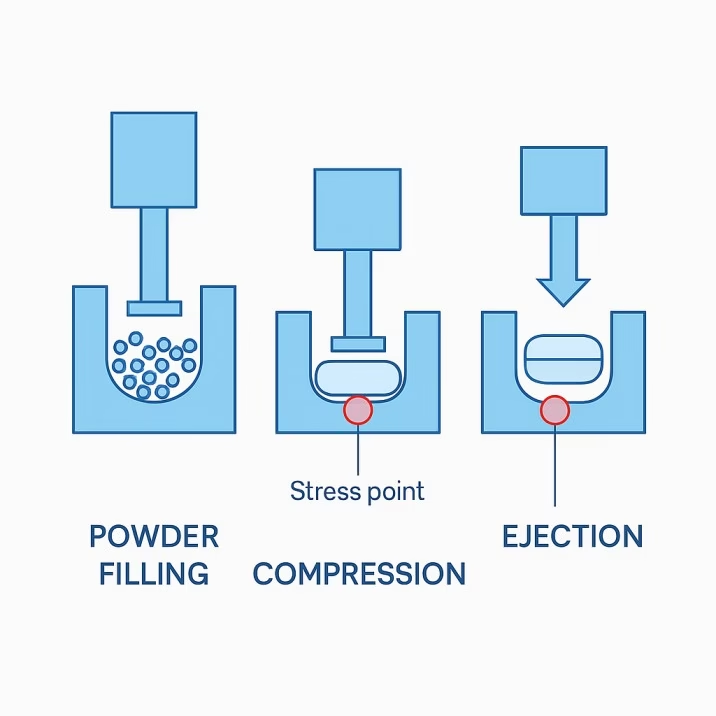
Tooling modifications frequently solve capping problems. Reducing cup depth by 10-20% decreases stress concentration while maintaining adequate tablet strength. Increasing cup radius provides more gradual stress transitions. Some applications benefit from beveled punch edges that eliminate sharp stress concentrations.
Lamination presents different challenges and solutions. This problem often stems from powder flow characteristics that create density variations within the tablet. Poor mixing can create layers of different materials that don’t bond properly. Electrostatic charging can cause powder segregation that leads to weak interfaces.
Process adjustments often resolve lamination issues more effectively than tooling changes. Improving powder blending homogeneity eliminates density variations. Anti-static agents reduce electrostatic effects that cause powder segregation. Pre-compression techniques can eliminate air entrapment that creates weak zones.
Weight Variation Troubleshooting
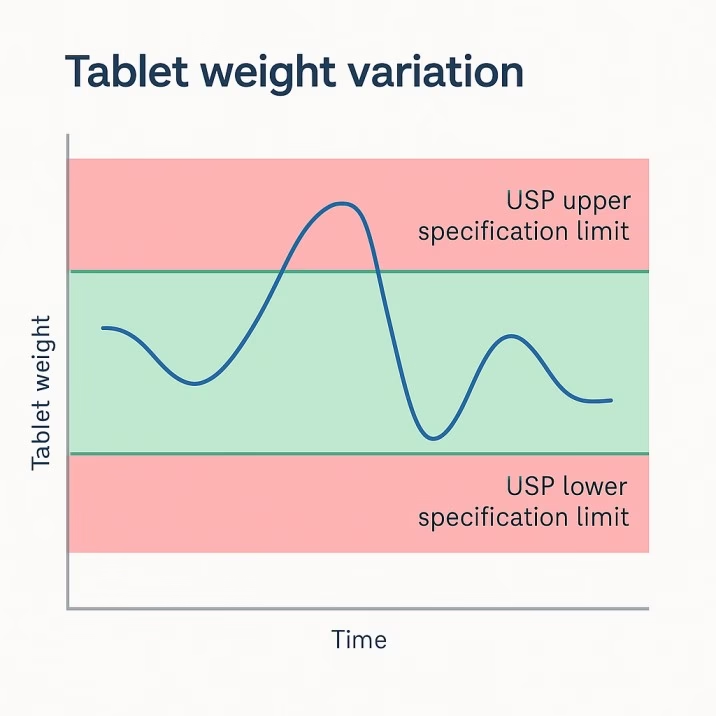
Weight variation problems frustrate pharmaceutical manufacturers because they directly impact regulatory compliance and product quality. USP specifications allow only ±5% variation for tablets over 324mg, with tighter tolerances for smaller tablets. Achieving these specifications requires understanding the complex interactions between tooling condition, powder characteristics, and process parameters.
Die wear represents the most common cause of progressive weight variation increases. As dies wear, the bore diameter increases and creates larger tablet diameters with higher weights. Bell-mouthing at the die entrance affects powder flow and creates position-dependent weight variations. Even microscopic wear patterns can cause measurable weight differences.

Punch length variations within sets create thickness-dependent weight differences. Manufacturing tolerances should keep punch length variations within ±0.013mm, but wear patterns can create larger differences over time. Thermal expansion during operation can also create temporary length variations that affect tablet weights.
Powder flow characteristics interact with die condition to create complex weight variation patterns. Free-flowing powders are less affected by minor die wear than cohesive materials. Particle size distribution changes can dramatically affect die filling characteristics. Even ambient humidity changes can alter powder flow and create weight variations.
The systematic approach to weight variation problems begins with data analysis to identify patterns. Random variations suggest powder flow issues, while systematic patterns indicate tooling problems. Position-dependent variations on rotary presses often point to specific worn dies or punch length differences.
Tooling inspection provides definitive diagnosis of wear-related problems. Pin gauges quickly identify oversized dies that require replacement. Precision measurement of punch lengths reveals sets that need reconditioning or replacement. Surface inspection often reveals wear patterns that explain weight variation trends.

Preventive measures provide the most cost-effective approach to weight variation control. Regular die inspection with pin gauges catches wear before it affects product quality. Punch set rotation ensures even wear patterns and extends service life. Environmental control minimizes humidity effects on powder flow characteristics.
Implementing Effective Maintenance and Lifecycle Management
Proper maintenance of tablet punches and dies represents one of the most cost-effective investments in pharmaceutical manufacturing. A systematic approach to tooling care can extend service life by 2-3 times while ensuring consistent product quality and regulatory compliance.
Daily Maintenance Excellence

Every production day should begin and end with systematic tooling inspection and care. Visual examination catches problems before they become costly failures. Punch faces should be completely free of sticking, picking, or embedded particles. Even microscopic deposits can grow into major problems if left unaddressed.
The cleaning process requires more sophistication than many manufacturers realize. Simply wiping punches with a rag can create scratches that become sticking initiation sites. Proper cleaning begins with appropriate solvent selection—USP-grade isopropyl alcohol works well for most applications, but some formulations require specialized cleaners.
Soft brush cleaning removes stubborn deposits without damaging precision surfaces. Natural bristle brushes work better than synthetic materials that can be too abrasive. The cleaning motion should follow the punch surface contours rather than cross-grain scrubbing that creates scratches.
Compressed air drying ensures complete moisture removal before storage. Water spots or residual solvents can cause corrosion or staining that affects subsequent production. The drying process should include all internal cavities and crevices where moisture can hide.
Documentation transforms maintenance from a chore into a valuable quality tool. Recording cleaning completion in batch records provides traceability for regulatory audits. Noting any unusual conditions or required repairs helps identify patterns that predict problems.
Weekly Deep Maintenance

Weekly maintenance provides opportunities for more thorough inspection and preventive care. Ultrasonic cleaning removes deposits that resist manual cleaning methods. The cavitation action reaches into microscopic surface irregularities where particles can hide.
Proper ultrasonic cleaning requires attention to solution selection, temperature control, and cleaning time. Pharmaceutical-grade cleaning solutions provide better results than generic alternatives. Temperature control around 40-50°C optimizes cleaning action without risking thermal damage. Cleaning times of 10-15 minutes typically provide optimal results without over-processing.
Detailed inspection during weekly maintenance catches problems early when corrections are still economical. Magnification reveals surface conditions invisible to naked-eye inspection. Systematically examining punch faces, die bores, and key areas identifies wear patterns before they affect product quality.
Precision measurement during weekly maintenance provides quantitative data for lifecycle planning. Pin gauges quickly check die bore dimensions for wear trends. Punch length measurement identifies sets approaching replacement criteria. Surface roughness spot checks verify that finish conditions remain within specifications.
The weekly maintenance cycle also provides opportunities for preventive actions. Light polishing can remove minor surface imperfections before they become major problems. Protective lubrication prevents corrosion during storage periods. Rotation of spare tooling sets ensures even utilization and extends overall service life.
Monthly Comprehensive Assessment

Monthly maintenance should include comprehensive evaluation of tooling condition and performance trends. This deeper analysis identifies subtle problems that daily and weekly inspections might miss while providing data for strategic replacement planning.
Wear pattern analysis during monthly inspections reveals important information about process conditions and formulation effects. Unusual wear patterns might indicate machine problems, formulation changes, or operator technique issues. Documenting these patterns helps optimize both tooling selection and process parameters.
Complete dimensional verification during monthly maintenance provides definitive data about tooling condition. All critical dimensions should be measured and compared to new tooling specifications. Tracking these measurements over time reveals wear rates and predicts replacement timing.
Performance trending analysis connects tooling condition to production metrics. Comparing defect rates, weight variation, and productivity data to tooling wear measurements identifies optimal replacement timing. This analysis often reveals that tooling replacement before complete wear-out provides better overall economics.
The monthly maintenance cycle should also include assessment of reconditioning opportunities. Many worn punches and dies can be restored to like-new condition through professional reconditioning services. This option typically costs 50-70% less than replacement while providing performance equal to new tooling.
Lifecycle Optimization Strategies
Effective lifecycle management requires systematic data collection and analysis to optimize both performance and costs. Understanding wear patterns, failure modes, and replacement timing transforms tooling management from reactive firefighting to proactive optimization.
Performance tracking should include both quantitative measurements and qualitative observations. Tablet count per tooling set provides basic productivity data, but defect rates and quality trends reveal performance degradation patterns. Maintenance frequency and downtime incidents indicate reliability trends that affect total costs.
Digital tracking systems provide sophisticated lifecycle management capabilities. RFID tags or barcode systems enable automatic data collection and analysis. Maintenance management software can track service history, predict replacement timing, and optimize inventory levels.
Cost analysis should include all lifecycle expenses, not just initial purchase prices. Tool replacement costs, maintenance expenses, quality impacts, and downtime losses all contribute to total tooling costs. Understanding these relationships enables better decision-making about premium tooling investments.
Retirement criteria should be based on total cost optimization rather than arbitrary wear limits. Sometimes retiring tooling before complete wear-out provides better economics through reduced maintenance costs and improved productivity. The optimal retirement point balances remaining tool value against increasing operating costs and quality risks.
Optimizing Costs and Maximizing ROI
The economics of tablet tooling extend far beyond initial purchase prices. A systematic approach to cost optimization can reduce total tooling expenses by 30-50% while improving quality and productivity. Understanding these economics enables better decisions about materials, suppliers, and replacement timing.
Understanding True Total Costs
Initial tooling costs represent only the tip of the economic iceberg. Standard B-type tooling might cost $500-1,500 per set, while premium D-type sets can reach $3,000-8,000 depending on materials and complexity. Custom geometry or specialized coatings can push costs even higher, but these upfront expenses often pale in comparison to operating costs over the tool’s lifetime.
Replacement frequency drives the largest component of long-term tooling costs. Standard tool steel punches might last 100,000-500,000 tablets before requiring replacement, while premium materials can extend this to 2,000,000-10,000,000 tablets. The math becomes compelling quickly—spending 5 times more for tooling that lasts 15 times longer provides obvious economic benefits.
Maintenance costs accumulate steadily throughout the tool’s service life. Regular cleaning, inspection, and reconditioning services typically cost $200-800 per service event. While these individual costs seem modest, they add up significantly over years of operation. Premium tooling often requires less frequent maintenance, reducing these accumulated costs.
Downtime represents the most expensive component of tooling economics for high-volume operations. Production line stoppage can cost $1,000-10,000 per hour depending on the facility and product value. Emergency tooling replacements, expedited shipping, and overtime labor all multiply these costs. Quality tooling that provides predictable service life enables planned maintenance during scheduled downtime.
Quality incidents create potentially catastrophic costs that are difficult to quantify but impossible to ignore. Rejected batches, regulatory investigations, and product recalls can cost hundreds of thousands or even millions of dollars. The right tooling selection and maintenance practices provide insurance against these devastating events.
Strategic Investment Analysis
The payback period calculation provides a simple framework for evaluating tooling investments. Premium tooling that costs $4,000 more than standard alternatives but saves $2,500 annually through reduced replacements and improved productivity pays for itself in 1.6 years. The remaining service life provides pure profit.
Net Present Value (NPV) analysis provides more sophisticated economic evaluation for long-term investments. Using standard pharmaceutical industry discount rates of 8-12%, most premium tooling investments show positive NPV over their service lives. The key lies in accurate estimation of cost savings and service life extension.
Sensitivity analysis reveals which variables most affect investment returns. Production volume changes typically have the greatest impact on tooling economics. Material costs, labor rates, and downtime expenses also significantly influence the analysis. Understanding these sensitivities helps identify where to focus optimization efforts.
Risk assessment adds another dimension to investment analysis. Premium tooling typically provides more predictable performance with lower failure risks. This predictability has value beyond simple cost calculations, particularly for critical products or high-volume operations where tooling failures create major disruptions.
Procurement Strategy Optimization
Volume purchasing provides immediate cost reductions for multi-product facilities. Annual contracts with committed volumes typically yield 10-20% discounts compared to spot purchases. Blanket purchase orders with scheduled releases provide similar benefits while maintaining inventory flexibility.
Supplier partnerships can provide benefits beyond simple price reductions. Long-term agreements often include service guarantees, technical support, and joint optimization programs. These relationships become particularly valuable when developing new products or solving challenging technical problems.
Standardization across product lines reduces complexity and costs throughout the organization. Using common tooling standards, materials, and suppliers simplifies procurement, inventory management, and operator training. The reduced variety also enables larger volume purchases with better pricing.
Group purchasing leverages the combined volumes of multiple facilities or business units. Even facilities in different geographic regions can often coordinate purchases to achieve better pricing and terms. Industry consortiums and buying groups provide similar benefits for smaller companies.
Performance-Based Contracting
Service Level Agreements (SLAs) shift risk from manufacturers to suppliers while aligning incentives for optimal performance. Guaranteed tool life commitments provide predictable costs and ensure supplier accountability for product quality. Performance bonuses reward suppliers for exceeding expectations.
Outcome-based pricing models tie supplier compensation to actual results rather than simply delivering products. Cost-per-tablet pricing aligns supplier interests with manufacturer productivity goals. Shared savings arrangements create win-win situations where both parties benefit from optimization improvements.
Risk-sharing arrangements provide protection against catastrophic failures while maintaining supplier incentives for quality. Penalty clauses ensure financial accountability for failures, while performance bonuses reward exceptional results. These arrangements work best with established supplier relationships and clear performance metrics.
Ensuring Regulatory Compliance and Quality Excellence
Pharmaceutical manufacturing operates in one of the most heavily regulated industries in the world. Tablet punches and dies must meet stringent requirements for materials, manufacturing processes, and quality systems. Understanding these requirements is essential for avoiding costly compliance problems and ensuring patient safety.

FDA and cGMP Fundamentals
The Food and Drug Administration’s current Good Manufacturing Practice (cGMP) regulations establish the foundation for pharmaceutical quality systems. Part 211 of Title 21 of the Code of Federal Regulations specifically addresses manufacturing, processing, packing, and holding of drugs. These regulations directly impact how tablet tooling must be specified, qualified, and maintained.
Equipment design and construction requirements demand that tablet tooling be suitable for its intended use and constructed from materials that don’t adversely affect product quality. This seemingly simple requirement has profound implications for material selection, surface finishes, and manufacturing processes. The tooling must not be reactive, additive, or absorptive under conditions of intended use.
Installation qualification provides documented verification that equipment is installed according to manufacturer specifications and regulatory requirements. For tablet tooling, this includes verification of dimensions, materials, surface finishes, and compatibility with existing equipment. The documentation must demonstrate that the tooling meets all specified requirements.
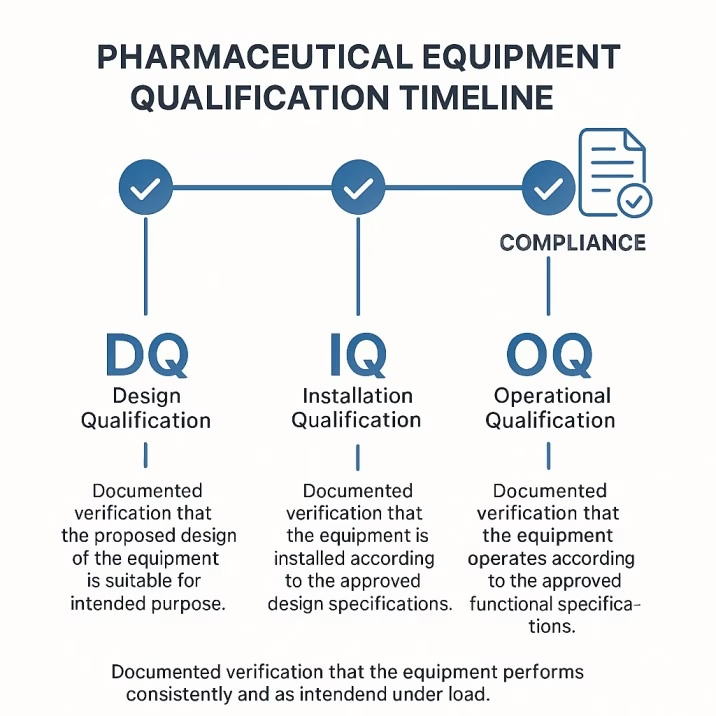
Operational qualification verifies that equipment operates according to operational requirements across anticipated operating ranges. For tooling, this includes demonstrating that tablets meet all quality specifications under normal operating conditions. The qualification must include worst-case scenarios and demonstrate system capabilities under stress conditions.
Performance qualification provides documented verification that the system consistently produces products meeting predetermined specifications. This phase requires extended operation under normal conditions with comprehensive product testing. The qualification must demonstrate consistent performance over time and across different operators.
Change Control and Documentation

Change control procedures ensure that modifications to equipment or processes don’t adversely affect product quality. For tablet tooling, changes in materials, suppliers, manufacturing processes, or specifications require systematic evaluation and approval. The complexity of change control often surprises manufacturers who assume that “equivalent” tooling doesn’t require documentation.
Risk assessment forms the foundation of effective change control. The assessment must consider potential impacts on product quality, process performance, and regulatory compliance. Low-risk changes might require only documentation, while high-risk modifications demand extensive testing and validation.
The evaluation process typically involves multiple disciplines including manufacturing, quality assurance, regulatory affairs, and technical development. Each group contributes expertise to assess potential impacts and determine necessary verification activities. This multidisciplinary approach helps identify risks that individual experts might miss.
Implementation of approved changes requires careful planning and documentation. Procedures must ensure that only approved modifications are implemented and that all affected personnel receive appropriate training. Parallel operation or extensive testing often provides confidence before full implementation.
Effectiveness checks verify that implemented changes achieve their intended benefits without creating unintended consequences. This monitoring continues for extended periods to ensure that long-term effects are captured and documented.

International Regulatory Harmonization
The International Council for Harmonisation (ICH) has developed guidelines that streamline regulatory requirements across major pharmaceutical markets. ICH Q7 addresses Good Manufacturing Practice for active pharmaceutical ingredients, while ICH Q8 focuses on pharmaceutical development approaches that can significantly impact tooling requirements.
Quality by Design (QbD) principles from ICH Q8 encourage systematic understanding of how formulation and process variables affect product quality. For tablet tooling, this means understanding relationships between tool materials, surface finishes, and geometric features with tablet quality attributes. This scientific approach often leads to more robust tooling specifications.
Design space concepts allow manufacturers to operate within proven acceptable ranges without requiring additional regulatory approval for changes. Establishing design space for tooling parameters provides operational flexibility while maintaining quality assurance. However, developing this understanding requires extensive characterization studies.
European Union regulations follow similar principles but include specific requirements that differ from FDA expectations. Annex 15 of the EU GMP guidelines addresses qualification and validation with particular emphasis on risk-based approaches. The European Medicines Agency (EMA) guidance documents provide additional clarity on expectations for equipment qualification.
Validation Protocol Development
Effective validation requires comprehensive protocols that address all aspects of tooling performance and qualification. Protocol development should begin during the tooling specification phase to ensure that all critical quality attributes are identified and addressed.
User Requirements Specifications (URS) document the intended use and performance expectations for tablet tooling. These specifications form the foundation for all subsequent qualification activities. The URS should address not only functional requirements but also regulatory compliance, cleaning procedures, and maintenance requirements.
Functional Requirements Specifications (FRS) translate user requirements into technical specifications that can be verified during qualification. For tooling, this includes dimensional tolerances, material specifications, surface finish requirements, and performance criteria. The FRS provides the basis for acceptance testing.
Design Qualification (DQ) verifies that the tooling design meets user requirements and applicable regulations. This phase reviews design documents, specifications, and supplier qualifications to ensure compliance before manufacturing begins. DQ provides an opportunity to catch problems before expensive tooling is produced.
Installation Qualification protocols must address all aspects of tooling installation and setup. This includes verification of dimensions, materials, surface finishes, and proper installation in tablet presses. The protocol should include acceptance criteria for all critical parameters and procedures for handling deviations.
Operational Qualification protocols verify that tooling operates correctly across the intended operating range. This includes testing under normal conditions as well as worst-case scenarios. The protocol should demonstrate that the tooling consistently produces tablets meeting all quality specifications.
Performance Qualification protocols provide documented evidence that the tooling consistently produces acceptable results under normal operating conditions. This phase typically requires extended operation with comprehensive product testing. The protocol should include statistical analysis of results and demonstration of process capability.
Quality Systems Integration
Modern pharmaceutical quality systems integrate tooling management with overall quality assurance programs. This integration ensures that tooling-related activities support broader quality objectives while maintaining regulatory compliance.
Risk management approaches help prioritize tooling activities based on potential impact on product quality and patient safety. High-risk tooling receives more intensive management and oversight, while routine applications can use simplified procedures. This risk-based approach optimizes resource utilization while maintaining appropriate control.
Supplier qualification programs ensure that tooling suppliers maintain appropriate quality systems and capabilities. Qualification typically includes facility audits, quality system assessments, and ongoing performance monitoring. Qualified supplier status enables simplified receiving procedures and reduced incoming inspection requirements.
Deviation and investigation procedures address tooling-related problems systematically. When tooling contributes to product quality problems, investigation procedures must identify root causes and implement effective corrective actions. The investigation process often reveals opportunities for improvement in tooling specifications or maintenance procedures.
Trending and analysis programs identify patterns that might not be obvious from individual events. Regular analysis of tooling performance data can reveal degradation trends, supplier issues, or process improvements. This proactive approach prevents problems rather than simply reacting to failures.
Embracing Future Trends and Industry 4.0 Technologies
The pharmaceutical industry stands at the threshold of a digital revolution that will transform tablet manufacturing and tooling management. Industry 4.0 technologies promise unprecedented visibility into tooling performance while enabling predictive maintenance and optimization strategies that were impossible just a few years ago.
Smart Tooling Revolution

The integration of sensors directly into tablet punches and dies represents one of the most exciting developments in recent years. These smart tools can monitor compression forces, temperatures, and vibration patterns in real-time, providing insights into both process conditions and tool health that were previously invisible.
Pressure sensors embedded in punch tips measure actual compression forces rather than relying on machine settings. This real-time data reveals force variations that indicate powder flow problems, die filling issues, or tool wear. The immediate feedback enables process adjustments before quality problems develop.
Temperature monitoring provides another window into process conditions and tool health. Excessive temperatures often precede sticking problems or indicate unusual friction from tool wear. Thermal patterns can also reveal uneven powder distribution or compression issues that affect tablet quality.
Vibration sensors detect early signs of tool wear, machine problems, or process disturbances. The characteristic vibration signatures of healthy operation enable automated detection of abnormal conditions. Machine learning algorithms can learn these patterns and provide increasingly sophisticated diagnostic capabilities.
Wireless data transmission enables real-time monitoring without compromising tool integrity or tablet press operation. Low-power sensors can operate for months on battery power while continuously streaming data to manufacturing execution systems. This connectivity transforms passive tools into active participants in process control.
Digital Twin Technology

Digital twin technology creates virtual models of tablet tooling and compression processes that mirror real-world performance in remarkable detail. These models enable virtual experimentation, optimization, and training without disrupting actual production.
Physics-based simulation models accurately predict compression forces, stress distributions, and tablet formation processes. These models help optimize punch cup geometries, predict tool life, and understand the effects of material property changes. Virtual testing significantly reduces the time and cost of new product development.
Wear prediction models use actual operating data to forecast tool life and optimal replacement timing. These models consider production volume, formulation characteristics, and operating conditions to provide accurate predictions. Manufacturers can plan maintenance activities and optimize inventory levels based on these predictions.
Process optimization through digital twins enables virtual testing of parameter changes without risking actual production. The models can predict the effects of compression force changes, speed adjustments, or formulation modifications. This capability accelerates process development and reduces the risk of costly experiments.
Training applications use digital twins to provide realistic operator training without consuming production time or materials. Operators can practice troubleshooting scenarios, maintenance procedures, and process optimization techniques in a safe virtual environment. This training improves operator competency while reducing risks to actual production.
Artificial Intelligence Applications

Machine learning algorithms are revolutionizing quality control and process optimization in tablet manufacturing. These systems can identify patterns and relationships that human operators might miss while providing increasingly sophisticated diagnostic and predictive capabilities.
Automated inspection systems use computer vision and machine learning to detect tablet defects with unprecedented accuracy and speed. These systems can identify surface imperfections, dimensional variations, and color differences with consistency that exceeds human capabilities. The immediate feedback enables real-time process adjustments.
Pattern recognition algorithms analyze vast amounts of process data to identify relationships between tooling conditions, process parameters, and product quality. These insights enable optimization strategies that would be impossible through traditional statistical methods. The algorithms continuously learn and improve their predictive capabilities.
Predictive maintenance systems analyze sensor data from smart tooling and tablet presses to predict failures before they occur. These systems provide 2-4 weeks advance warning of tool failures, enabling planned maintenance during scheduled downtime. The cost savings from avoiding unplanned downtime often justify the technology investment within months.
Process control applications use artificial intelligence to continuously optimize tablet manufacturing parameters. The systems can adjust compression forces, speeds, and environmental conditions to maintain optimal quality while maximizing productivity. This automated optimization often achieves performance levels that exceed manual control.
Sustainability and Circular Economy
Environmental considerations are becoming increasingly important in pharmaceutical manufacturing, driving innovations in tooling materials, manufacturing processes, and lifecycle management approaches that reduce environmental impact while often improving economics.
Remanufacturing programs restore worn tooling to like-new condition using advanced machining and coating technologies. These programs typically achieve 90-95% of new tool performance while using 60-70% less energy and materials than manufacturing new tools. The cost savings of 40-60% compared to new tooling make these programs attractive for both economic and environmental reasons.
Material recovery initiatives reclaim valuable materials like tungsten, chromium, and specialty steels from worn tooling. Advanced recycling processes can recover these materials with minimal quality loss, reducing the need for virgin materials. The recovered materials often find applications in new tooling or other industrial uses.
Design for recyclability principles influence new tooling development to facilitate end-of-life material recovery. These design approaches consider material selection, joining methods, and coating systems that enable efficient separation and recovery. The additional design effort typically pays dividends through reduced disposal costs and material credits.
Closed-loop systems integrate tooling suppliers, remanufacturing services, and material recovery operations to create circular material flows. Manufacturers can participate in take-back programs where suppliers accept worn tooling in exchange for credits toward new purchases. These systems optimize material utilization while reducing waste streams.
Green manufacturing technologies reduce the environmental impact of tooling production through improved processes and energy sources. Water-based coating processes eliminate organic solvents, while renewable energy powers manufacturing operations. These improvements often provide operational benefits beyond environmental advantages.
Mastering Supplier Selection and Procurement
The success of your tablet manufacturing operation depends heavily on selecting the right tooling suppliers and managing those relationships effectively. The complexity of modern pharmaceutical manufacturing demands suppliers who understand not just machining and materials, but also regulatory requirements, quality systems, and the unique challenges of pharmaceutical production.
Building Your Supplier Evaluation Framework
Technical capability assessment forms the foundation of effective supplier evaluation. The best prices mean nothing if the supplier can’t deliver tooling that meets your performance requirements. Manufacturing capabilities should include advanced CNC machining centers, electrical discharge machining (EDM) for complex geometries, and sophisticated measurement equipment for quality verification.

Look for suppliers with comprehensive quality management systems that go beyond basic ISO 9001 certification. ISO 13485 certification for medical device manufacturing demonstrates understanding of pharmaceutical quality requirements. FDA device establishment registration shows commitment to regulatory compliance, while AS9100 aerospace certification often indicates exceptional quality capabilities.

Engineering support capabilities distinguish excellent suppliers from simple manufacturers. The best suppliers provide design optimization recommendations, problem-solving assistance, and application expertise that helps you achieve better results. They should understand powder compression physics, formulation effects on tooling performance, and how to optimize designs for your specific requirements.
Material science expertise becomes crucial when dealing with challenging formulations or specialized applications. Your supplier should understand the relationships between steel grades, heat treatments, surface coatings, and performance characteristics. They should be able to recommend material solutions for specific problems and explain the technical rationale behind their recommendations.
Innovation capability indicates whether the supplier will help you stay competitive as technology evolves. Look for suppliers who invest in research and development, participate in industry conferences, and introduce new technologies proactively. These suppliers become partners in your success rather than simply vendors who respond to purchase orders.
Due Diligence and Risk Assessment
Financial stability assessment protects you from supply disruptions caused by supplier business problems. Credit ratings from Dun & Bradstreet or similar services provide objective assessments of financial health. Annual revenue, profitability trends, and debt ratios reveal business sustainability. Don’t overlook succession planning and management depth, particularly for smaller specialized suppliers.
Supply chain resilience has become increasingly important as global disruptions affect material availability and transportation. Evaluate suppliers’ raw material sourcing strategies, geographic diversification, and inventory management approaches. Suppliers with multiple manufacturing locations and diverse supplier bases provide better protection against disruptions.
Operational excellence indicators predict whether suppliers can consistently meet your delivery and quality requirements. On-time delivery performance should consistently exceed 95%, while quality metrics should demonstrate continuous improvement trends. Capacity utilization analysis reveals whether suppliers can handle your growth or respond to urgent requirements.
Business continuity planning addresses potential disasters or disruptions that could affect your supply. Suppliers should have documented plans for natural disasters, pandemic responses, and cyber security incidents. Insurance coverage for professional liability and product liability provides additional protection for your operations.
Strategic Procurement Approaches

Single source strategies work well when you find exceptional suppliers who provide superior value through technical expertise, quality performance, or innovation capabilities. The closer relationship enables better communication, joint development projects, and customized solutions. However, single sourcing increases supply risk and potentially reduces negotiating leverage.
Dual source strategies balance supply security with competitive pricing while maintaining manageable complexity. The typical 70/30 or 60/40 allocation provides the primary supplier with sufficient volume for good pricing while maintaining an active backup supplier. Success requires consistent specifications and quality standards across both suppliers.
Multiple source strategies maximize competition and supply flexibility but significantly increase management complexity. This approach works best for high-volume, standardized products where suppliers can easily substitute for each other. Quality considerations become paramount when multiple suppliers produce identical products.
Partnership development transforms transactional relationships into strategic alliances that benefit both parties. Joint development projects, shared technology roadmaps, and collaborative problem-solving create win-win situations. These relationships often produce innovations and cost reductions that benefit everyone involved.
Contract Optimization and Risk Management
Pricing model selection significantly impacts both costs and supplier relationships. Fixed pricing provides budget predictability but may not reflect market conditions or supplier cost changes. Cost-plus models provide transparency but require more management oversight. Market-based pricing adjusts to competitive conditions but creates budget uncertainty.
Performance-based contracts align supplier incentives with your operational requirements. Service level agreements should specify delivery performance targets, quality standards, and response time requirements. Performance bonuses reward exceptional results while penalty clauses ensure accountability for failures.
Risk management clauses protect both parties from unforeseeable circumstances while establishing clear responsibilities. Force majeure provisions address natural disasters and other uncontrollable events. Change management procedures ensure that modifications to specifications or requirements are handled systematically.
Intellectual property protection becomes crucial when sharing proprietary information or developing custom solutions. Confidentiality agreements protect sensitive information while invention assignment clauses clarify ownership of jointly developed technologies. Patent indemnification provisions protect against infringement claims.
Your Path to Tablet Tooling Excellence
The journey from basic understanding to mastery of tablet punches and dies requires systematic application of the principles and practices outlined in this comprehensive guide. Success comes not from implementing every suggestion simultaneously, but from prioritizing improvements based on your specific needs and capabilities.
Immediate Action Steps
Start with a comprehensive audit of your current tooling inventory and performance metrics. Document what tooling you’re using, how it’s performing, and where problems occur most frequently. This baseline assessment provides the foundation for all improvement efforts and helps prioritize investments for maximum impact.
Implement systematic maintenance procedures if they don’t already exist. The daily, weekly, and monthly maintenance protocols described earlier will immediately improve tooling performance and extend service life. These procedures cost almost nothing to implement but often provide dramatic improvements in consistency and productivity.
Evaluate your supplier relationships using the framework provided in this guide. Identify which suppliers meet your current and future needs while noting areas where improvements are needed. This assessment might reveal opportunities for supplier consolidation, partnership development, or new supplier qualification.
Medium-Term Strategic Initiatives
Develop comprehensive tooling specifications that address not just dimensional requirements but also material properties, surface finishes, and performance expectations. These specifications become the foundation for supplier selection, quality control, and problem resolution. Involve multiple disciplines in specification development to ensure all requirements are captured.
Implement data collection and analysis systems that provide visibility into tooling performance trends. Simple spreadsheet tracking often provides sufficient capabilities initially, while more sophisticated manufacturing execution systems can be added as needs grow. The key is consistent data collection and regular analysis for improvement opportunities.
Establish supplier partnerships with your most critical vendors. These relationships should include regular performance reviews, joint improvement projects, and collaborative problem-solving. The investment in relationship development typically pays dividends through improved service, better pricing, and access to new technologies.
Long-Term Vision and Capabilities
Plan for Industry 4.0 integration as smart tooling technologies become more widely available and cost-effective. Start with pilot projects that demonstrate value before committing to full-scale implementations. The early experience will help you understand the benefits and challenges while building organizational capabilities.
Develop internal expertise through training programs, industry participation, and collaboration with suppliers and equipment manufacturers. The pharmaceutical industry is rapidly evolving, and staying current requires continuous learning and adaptation. Invest in your people to build the capabilities needed for future success.
Consider sustainability initiatives that reduce environmental impact while often improving economics. Remanufacturing programs, material recovery initiatives, and green manufacturing technologies provide both environmental and economic benefits. Organizations like the International Society for Pharmaceutical Engineering (ISPE) provide resources and best practices for implementing sustainable manufacturing approaches.
Measuring Success and Continuous Improvement
Establish key performance indicators that align with your business objectives and track them consistently. Typical metrics include tooling cost per tablet produced, unplanned downtime due to tooling issues, quality defect rates related to tooling, and overall equipment effectiveness. These metrics provide objective measures of improvement and identify areas needing attention.
Implement regular review processes that analyze performance trends and identify improvement opportunities. Monthly reviews typically provide sufficient frequency for most operations, though high-volume facilities might benefit from more frequent analysis. Include multiple perspectives in these reviews to ensure comprehensive evaluation.
Benchmark your performance against industry standards and best practices. Industry associations like the American Association of Pharmaceutical Scientists (AAPS) and the Parenteral Drug Association (PDA) provide opportunities to understand how others achieve superior performance through conferences, training programs, and peer networks. These insights often reveal improvement opportunities that internal analysis might miss.
The Competitive Advantage of Excellence
Mastering tablet punches and dies provides competitive advantages that extend far beyond simple cost reduction. Superior tooling management enables faster new product introduction, higher quality consistency, improved regulatory compliance, and greater operational flexibility. These capabilities become increasingly valuable as the pharmaceutical industry faces growing pressures for efficiency and quality.
The pharmaceutical industry rewards organizations that consistently deliver high-quality products at competitive costs. Excellence in tooling management contributes to both objectives while providing the reliability and consistency that regulatory agencies and customers demand. The investment in tooling excellence pays dividends throughout the organization.
Innovation in tooling management often leads to broader process improvements and competitive advantages. Organizations that master these fundamentals are better positioned to adopt new technologies, enter new markets, and respond to competitive pressures. The capabilities developed through tooling excellence transfer to other areas of manufacturing and business operations.
Your Journey to Tooling Mastery Begins Now
The world of tablet punches and dies may seem complex and technical, but the principles of success are straightforward: understand your requirements, select appropriate solutions, maintain them properly, and continuously improve your capabilities. This guide has provided the roadmap—now it’s time to begin your journey.
The pharmaceutical industry will continue evolving rapidly, driven by new technologies, changing regulations, and increasing quality expectations. Organizations that master the fundamentals of tablet tooling management will be best positioned to adapt and thrive in this changing environment. The investment in excellence pays dividends not just in improved performance, but in organizational capabilities that enable long-term success.
Whether you’re just beginning to explore tablet tooling optimization or looking to take your existing programs to the next level, the principles and practices outlined in this guide provide a proven path to success. The journey requires commitment and systematic application, but the rewards—improved quality, reduced costs, enhanced productivity, and competitive advantage—justify the effort.
Start where you are, use what you have, and do what you can. Every improvement, no matter how small, moves you closer to tooling excellence and manufacturing success. The tablet punches and dies in your facility represent more than just tools—they’re the foundation of your quality, productivity, and competitive position in the pharmaceutical marketplace.
Your journey to tooling mastery begins with the next tablet you produce. Make it count.
Ready to transform your tablet manufacturing operation? Contact our technical specialists for a comprehensive assessment of your tooling needs and customized recommendations for improvement. With decades of experience in pharmaceutical tooling, we’re here to help you achieve excellence in every aspect of tablet production.
Get Expert Guidance:
- Schedule a technical consultation for your specific requirements
- Request competitive quotes for premium tooling solutions
- Access emergency support for critical production issues
- Join our training programs for operator and maintenance excellence
Transform your pharmaceutical manufacturing with the right tooling strategy, expert guidance, and proven solutions. Your success is our mission.
Related Articles
Essential Reading for Tablet Manufacturing Excellence:
Tooling Selection & Specifications
- TSM vs EU Standards: Complete Comparison Guide for Global Manufacturing
- How to Choose the Right Tablet Punches for Your Formulation
- Custom vs Standard Tooling: Decision Framework and Cost Analysis
- Multi-Tip Tooling: Productivity Enhancement Guide for High-Volume Production
Troubleshooting & Problem Solving
- Tablet Sticking Solutions: Complete Troubleshooting Guide
- Preventing Capping and Lamination: Technical Solutions That Work
- Weight Variation Problems: Root Cause Analysis and Solutions
- Common Tablet Press Problems and Expert Solutions
Maintenance & Optimization
- Pharmaceutical Tooling Maintenance: 7-Step Process for Maximum Life
- Predictive Maintenance for Tablet Compression Equipment
- ROI Calculator: Tablet Tooling Investment Analysis
- Quality Control: Testing and Validation Procedures
Advanced Topics
- Materials Science: Steel Grades and Coatings Explained
- Industry 4.0 Integration: Smart Tooling Solutions
- Regulatory Compliance: FDA Validation Requirements
- Supplier Evaluation: Complete Procurement Guide
Industry Applications
- Generic Drug Manufacturing: Tooling Considerations
- Small Batch Production: R&D Tooling Solutions
- Continuous Manufacturing: Tooling Implications
- Pharmaceutical vs Nutraceutical: Tooling Requirements
Explore our comprehensive library of technical resources to master every aspect of tablet compression tooling and pharmaceutical manufacturing excellence.